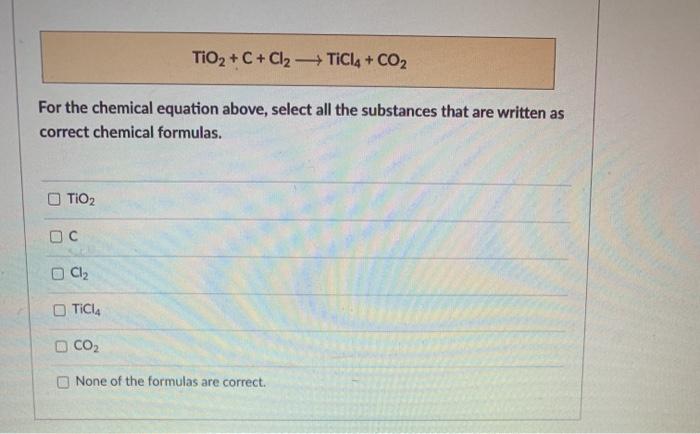
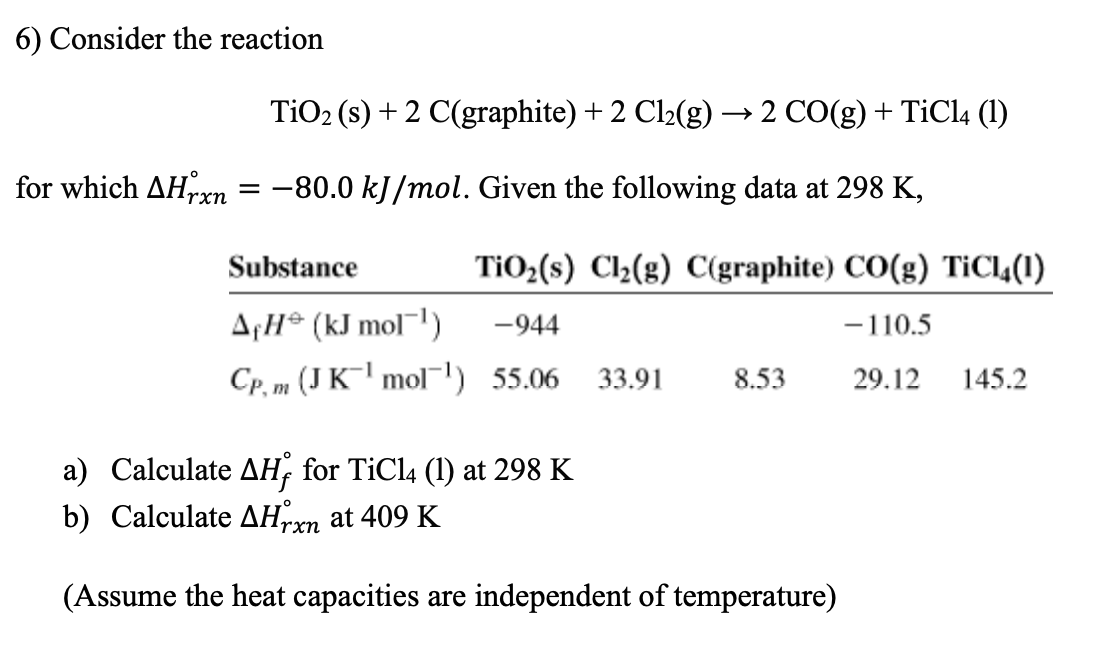
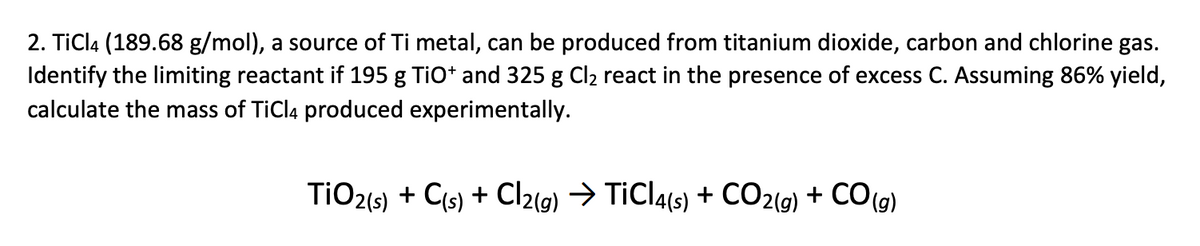
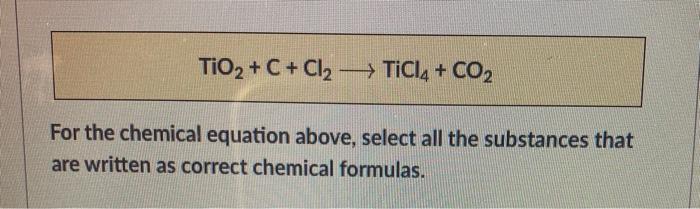
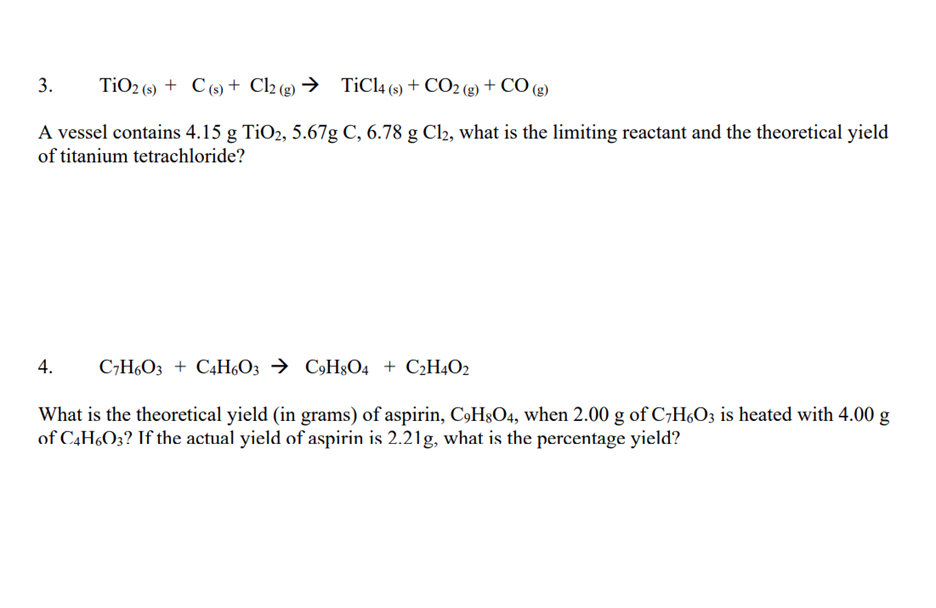TiO2 +C+2Cl2 →TiCl4 +CO2 What mass of Cl2 is needed to react with 1.25 mol TiO2? What mass of C is needed to react with 1.252 mol TiO2? - GuideChem

Titanium, which is used to make airplane engines and frames can be obtained from titanium tetrachloride, which in turn is obtained from titanium oxide by the following process: 3TiO2(s) + 4C(s) +

Insight into the performance of UV/chlorine/TiO2 on carbamazepine degradation: The crucial role of chlorine oxide radical (ClO•) - ScienceDirect

SOLVED: Consi der the following reaction; 2 TiO2 3C+4Cl2 7 2 TiCly CO2 2 CO If 12 moles TiO2, moles €, and 10 moles Clz are reacted, the limiting reagent will be Cl2 CO2 TiO2

SOLVED: QUESTION Consider the following eaction: 2 Ti02 +3C+4Cl2 2 TiCl4 CO2 2 CO If 12 moles TiO2' moles C, and 10 moles Clz are reacted, the limiting reagent will be CO2
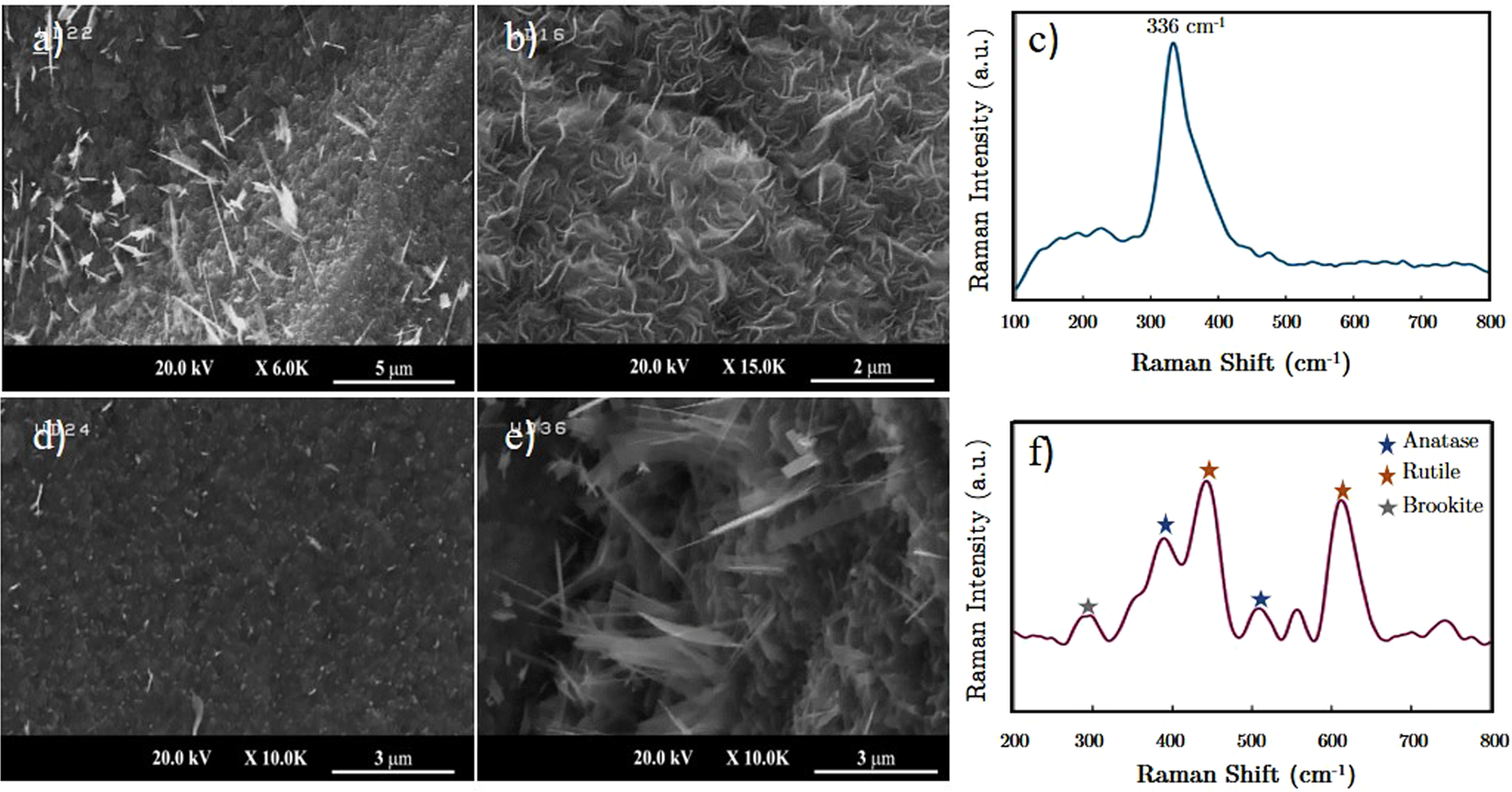
Evolution of large area TiS2-TiO2 heterostructures and S-doped TiO2 nano-sheets on titanium foils | Scientific Reports

Sensors | Free Full-Text | Effect of Pd-Sensitization on Poisonous Chlorine Gas Detection Ability of TiO2: Green Synthesis and Low-Temperature Operation
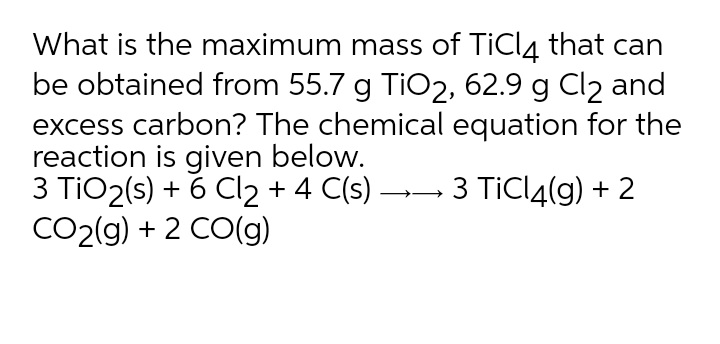
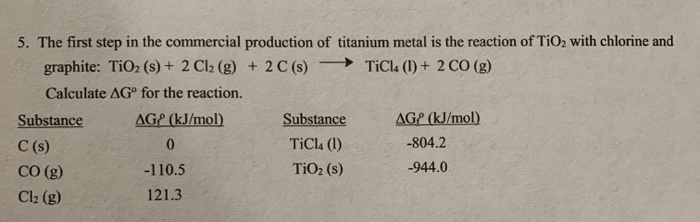
SOLVED: Titanium(IV) chloride can be be prepared from titanium(IV) oxide, carbon and chlorine gas according to the following chemical equation: 3TiO2(s) + 4 C(s) + 6 Cl2(g) ⟶ 3 TiCl4(l) + 2

![Solved] . Homework - Week 9 QUESTION 1. Balance each equation. a. Ni(s) +... | CliffsNotes Solved] . Homework - Week 9 QUESTION 1. Balance each equation. a. Ni(s) +... | CliffsNotes](https://coursehero.s3.amazonaws.com/qattachments_aeaacc4dafff152758f37a85c243201fb41ca0c1.png?X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIAIAYW2E6VOLDTI35A%2F20230329%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Date=20230329T191552Z&X-Amz-SignedHeaders=host&X-Amz-Expires=60&X-Amz-Signature=b57c163233b054114b34ba2b1b0ca402f4e6c43f0fda005b5a1ad867642bc4bd)