Titanium, which is used to make airplane engines and frames can be obtained from titanium tetrachloride, which in turn is obtained from titanium oxide by the following process: 3TiO2(s) + 4C(s) +

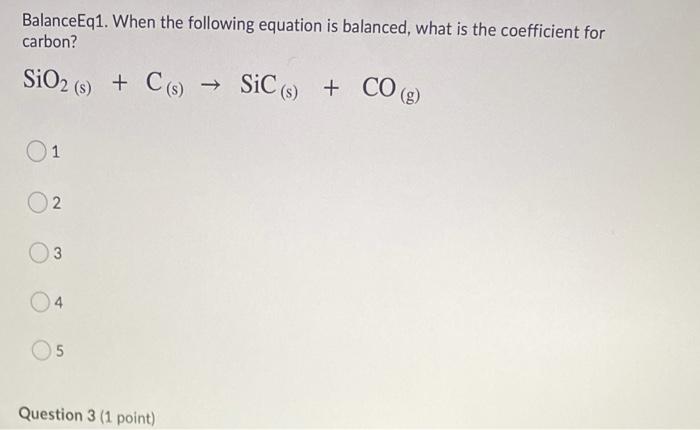
Silicon carbide, SiC,is produced by heating SiO(2) and C to high temperature according to the equation: SiO(2)(g) + 3C(s) rArr SiC(s) + 2CO(g) How many grams of SiC could be formed by
Effects of oxidation of SiC aggregates on the sintering behaviour and microstructures of SiC-CaAl O composite refractories
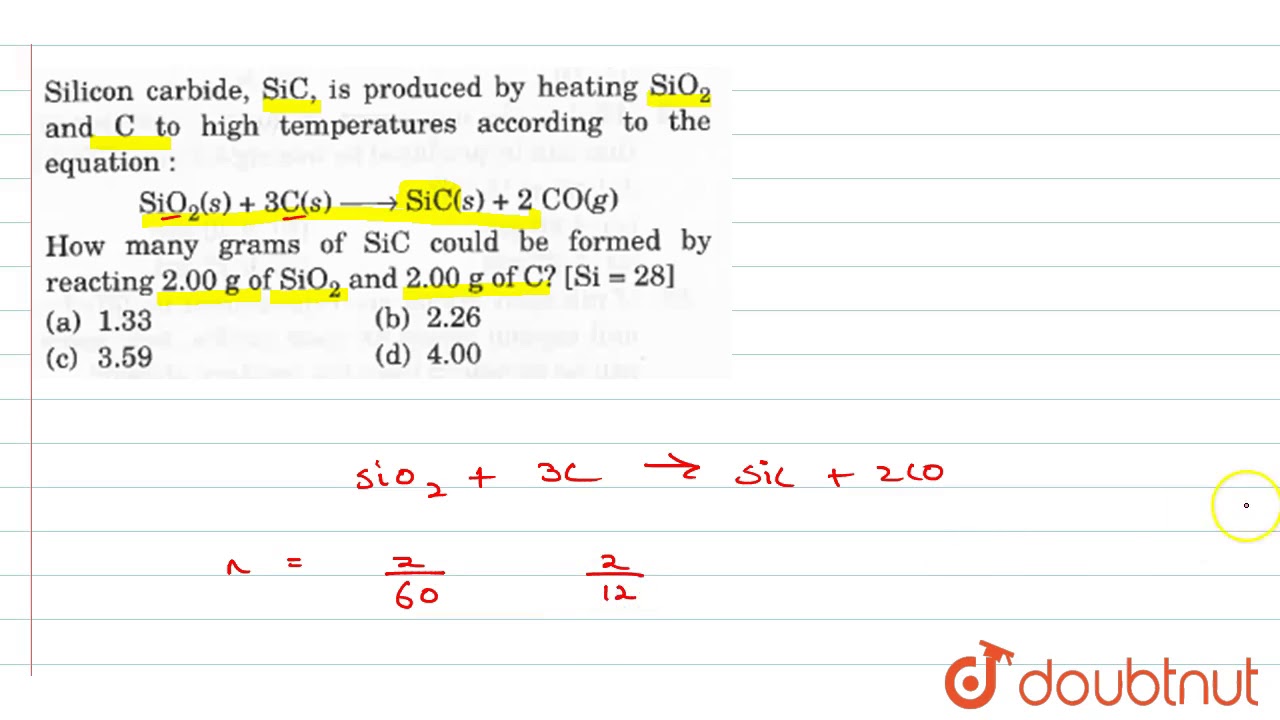
Silicon carbide, SiC,is produced by heating `SiO_(2)` and C to high temperature according to the - YouTube

Stoichiometry Notes Chemistry Mrs. Stoops. Stoichiometry Notes Converts grams of one substance into a different substance. Uses: Balanced chemical equations. - ppt download

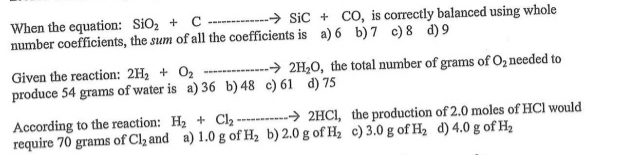
SOLVED: Silicon carbide; an abrasive, is made by the reaction of silicon dioxide with graphite: hcat SiOz + C SiC + CO (balanced ?) We mix 280. g of SiOz and 217

SOLVED: Silicon carbide, an abrasive, is made by the reaction of silicon dioxide with graphite. heat SiOz + C SiC CO (balanced 2) We mix 270. g of SiOz and 207 g

SOLVED: Silicon carbide; an abrasive; is made by the reaction of silicon dioxide with graphite. heat SiO2 + C SiC + CO (balanced ?) We mix 290. g of SiOz and 217

From the following data at 25C, Calculate the standard enthalpy of formation of FeO(s) and of Fe2O3 (s) .Reaction ΔrH (KJ/mole) (1) Fe2O3 (s) + 3C (graphite) → 2Fe (s) + CO (

From the following data at 25C, Calculate the standard enthalpy of formation of FeO(s) and of Fe2O3 (s) .Reaction ΔrH (KJ/mole) (1) Fe2O3 (s) + 3C (graphite) → 2Fe (s) + CO (
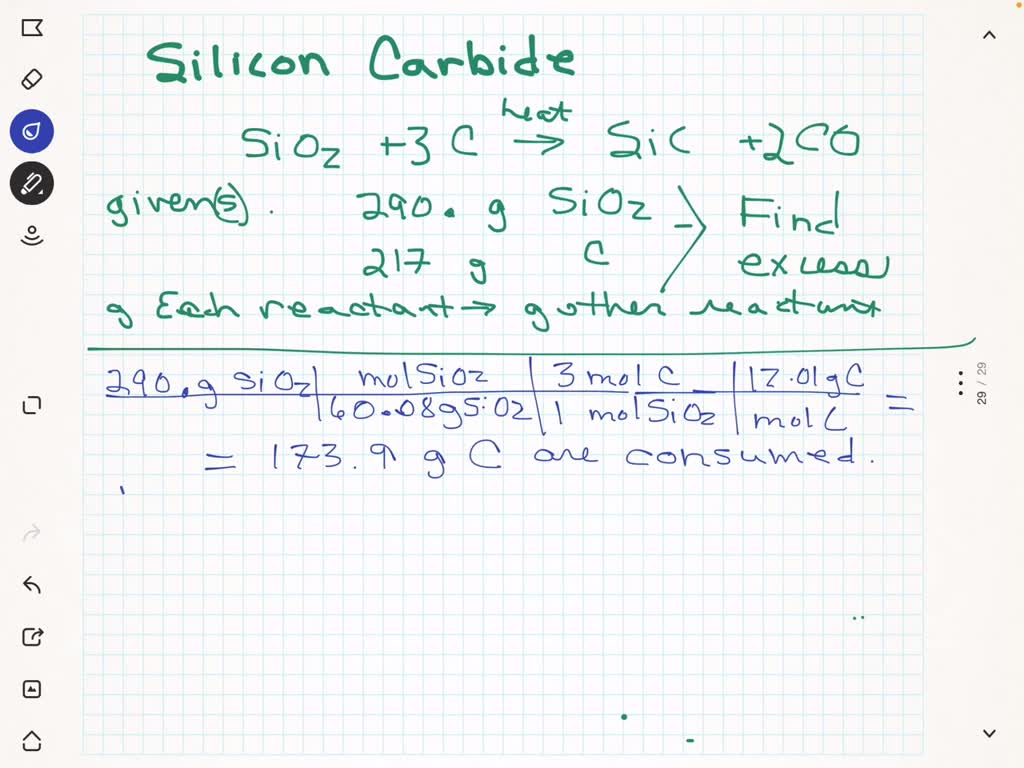
SOLVED: Silicon carbide; an abrasive; is made by the reaction of silicon dioxide with graphite. heat SiO2 + C SiC + CO (balanced ?) We mix 290. g of SiOz and 217

From the thermo chemical reactions, C(graphite) + 1/2O2→ CO;Δ H = - 110.5KJ CO + 1/2O2→ CO2;Δ H = - 283.2KJ. Δ H for the reaction, C(graphite) + O2→ CO2 is:
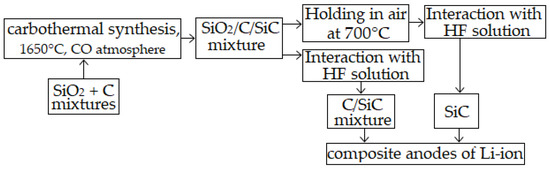
Applied Sciences | Free Full-Text | Synthesis of C/SiC Mixtures for Composite Anodes of Lithium-Ion Power Sources

Stoichiometry Notes Chemistry Mrs. Stoops. Stoichiometry Notes Converts grams of one substance into a different substance. Uses: Balanced chemical equations. - ppt download
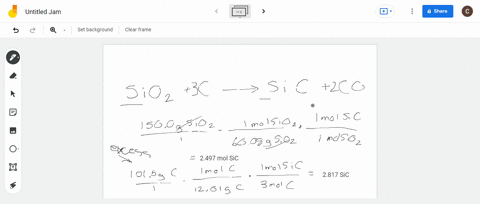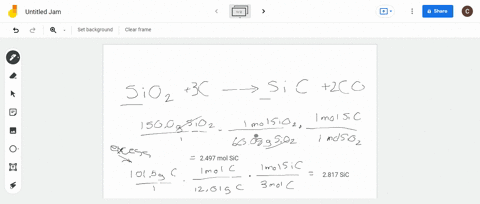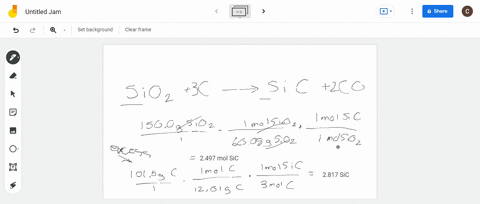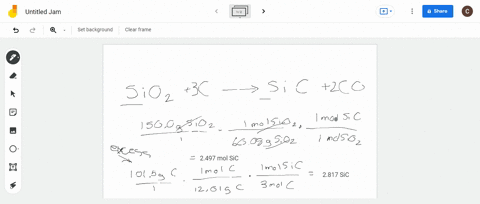
SOLVED:Silicon carbide, an abrasive, is made by the reaction of silicon dioxide with graphite. SiO2+C heat ⟶ SiC+CO (balanced?) We mix 150.0 g of SiO2 and 101.5 g of C. If the