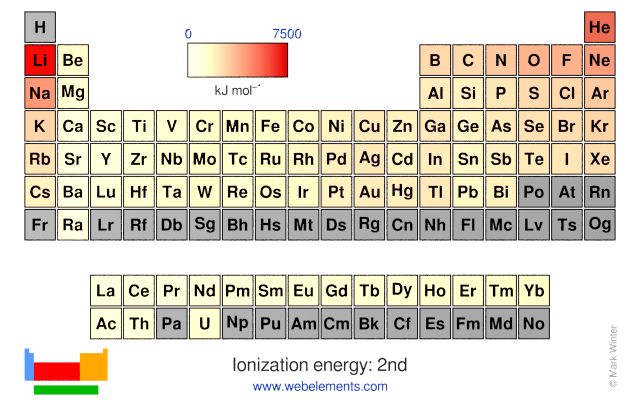
The calculation of the second ionization energies from the values of... | Download Scientific Diagram
The correct order of decreasing second ionization energy of Li, Be, Ne,(1) Ne>B>Li>C>BeC, B(2) Li>Ne C>B>Be(3) Ne>C>B>Be>Li4)
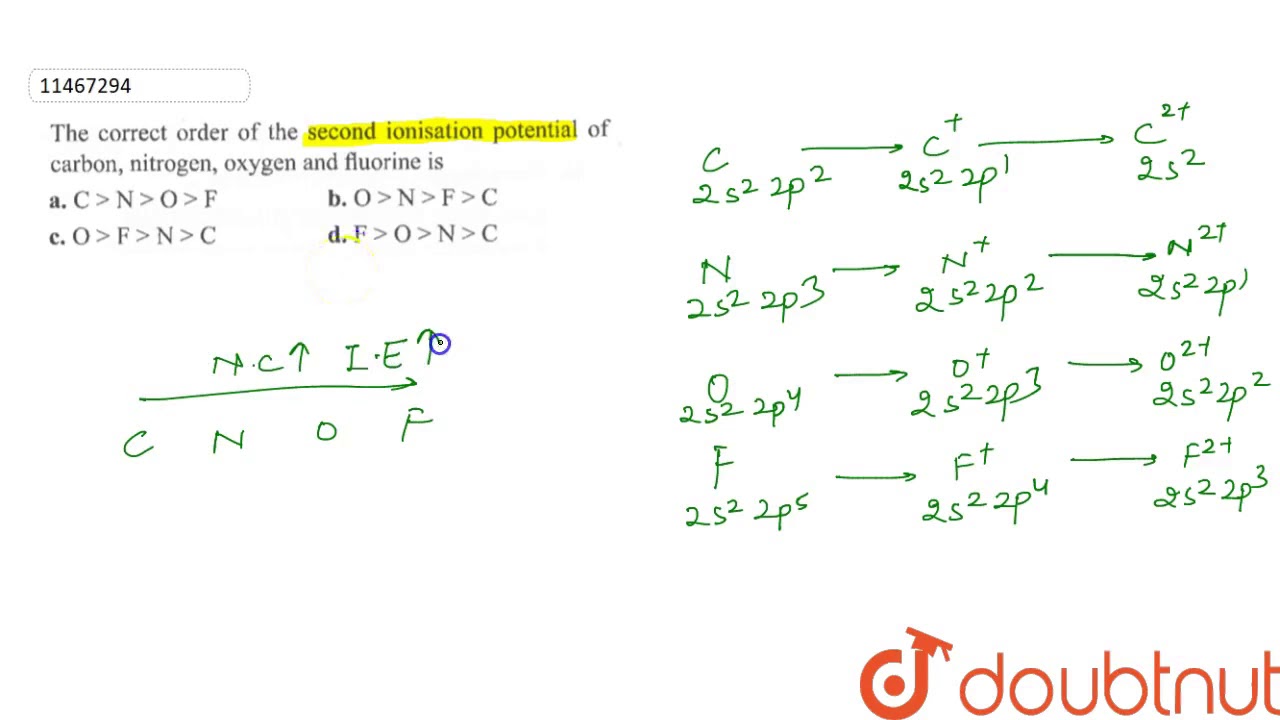
The correct order of the second ionisation potential of carbon, nitrogen, oxygen and fluorine is - YouTube

Ionisation energies of Na and Al are respectively 495.8 kJ/mol and 571.77 kJ/mol . What should be the ionisation energy of Mg?

Difference Between First and Second Ionization Energy (I1E vs I2E) | Compare the Difference Between Similar Terms
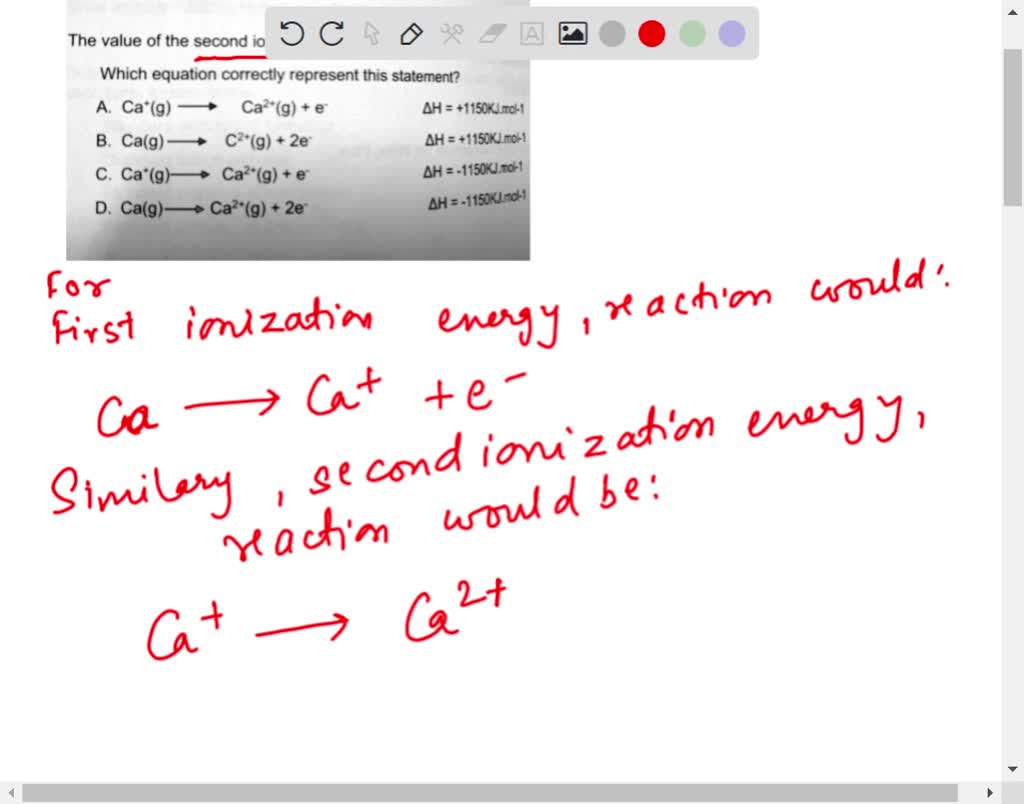
SOLVED: The value of the second ionisation energy of calcium is 1150 Klmol1 Which equation correctly represent this statement? A Ca (g) Ca?*(g) + e AH = +1150KJ,mol:1 B. Ca(g) c*(g) -
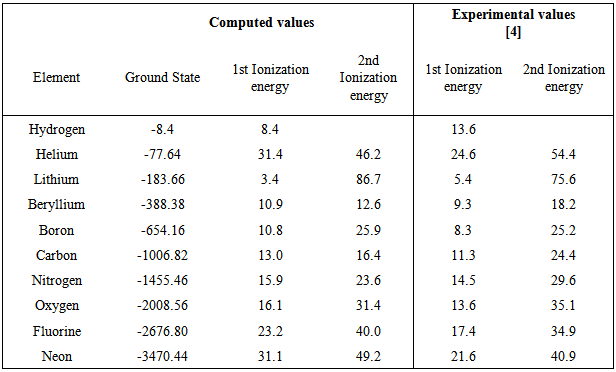
Computation of the First and Second Ionization Energies of the First Ten Elements of the Periodic Table Using a Modified Hartree-Fock Approximation Code
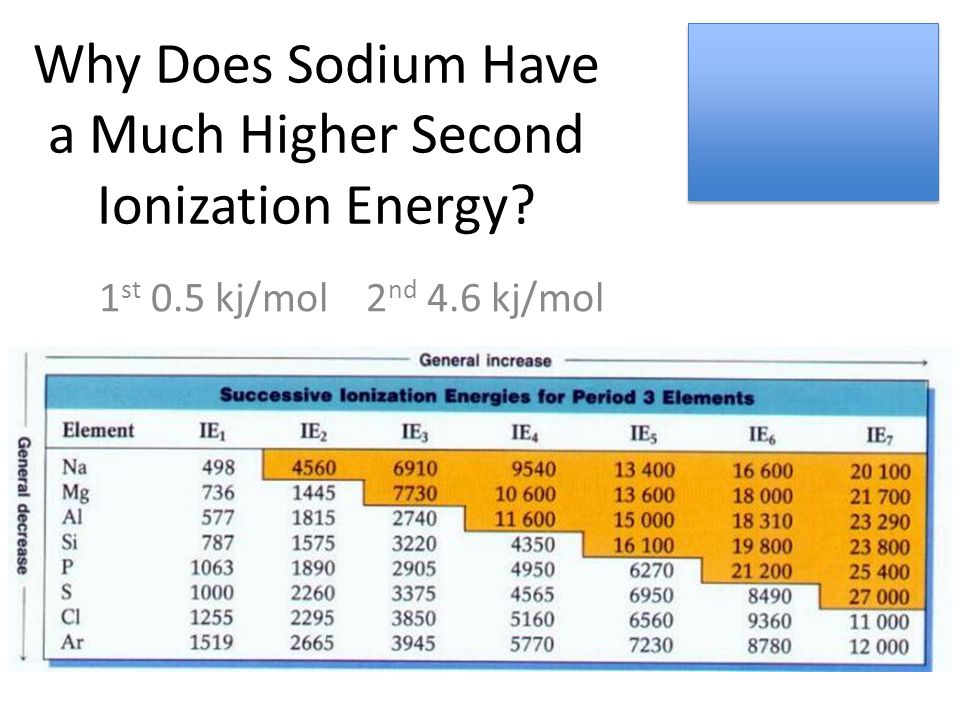
Why Does Sodium Have a Much Higher Second Ionization Energy? 1 st 0.5 kj/mol 2 nd 4.6 kj/mol. - ppt download
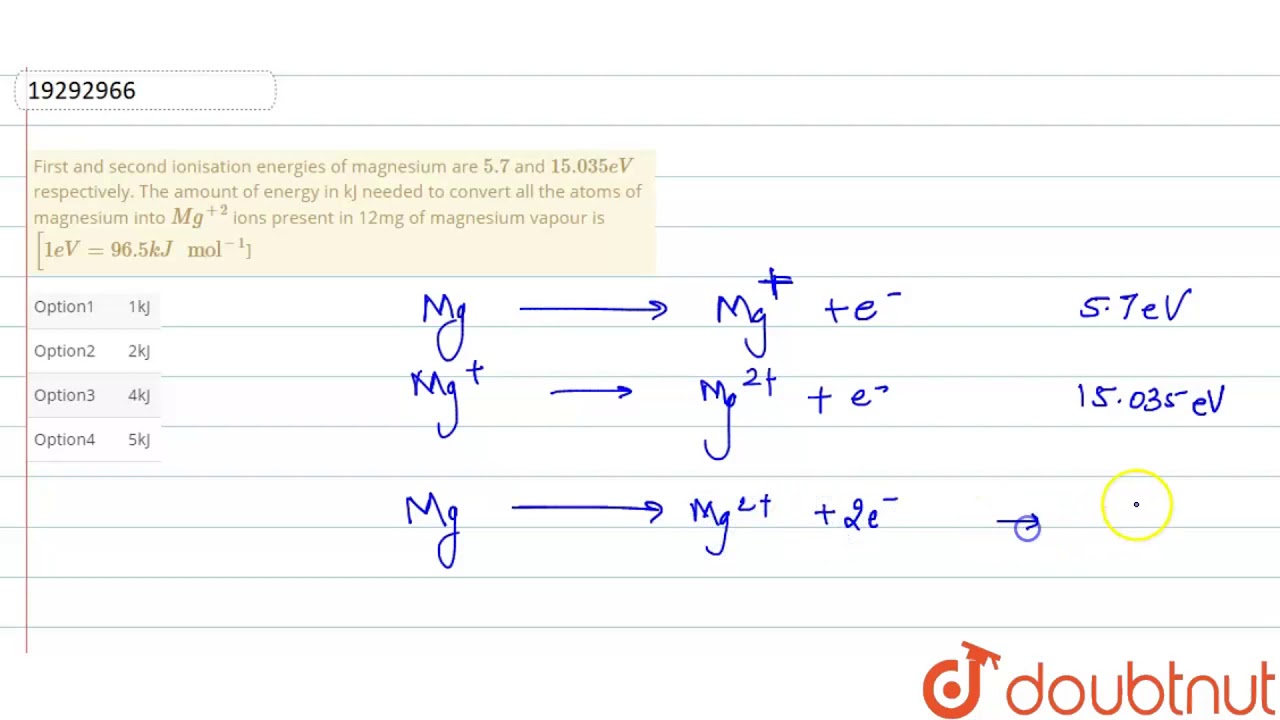
First and second ionisation energies of magnesium are `5.7` and `15.035eV` respectively. The amount - YouTube

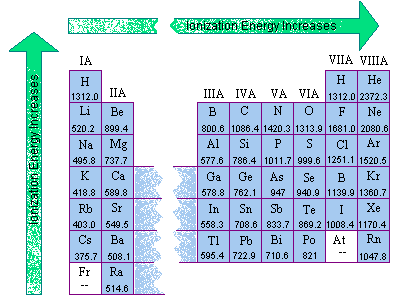
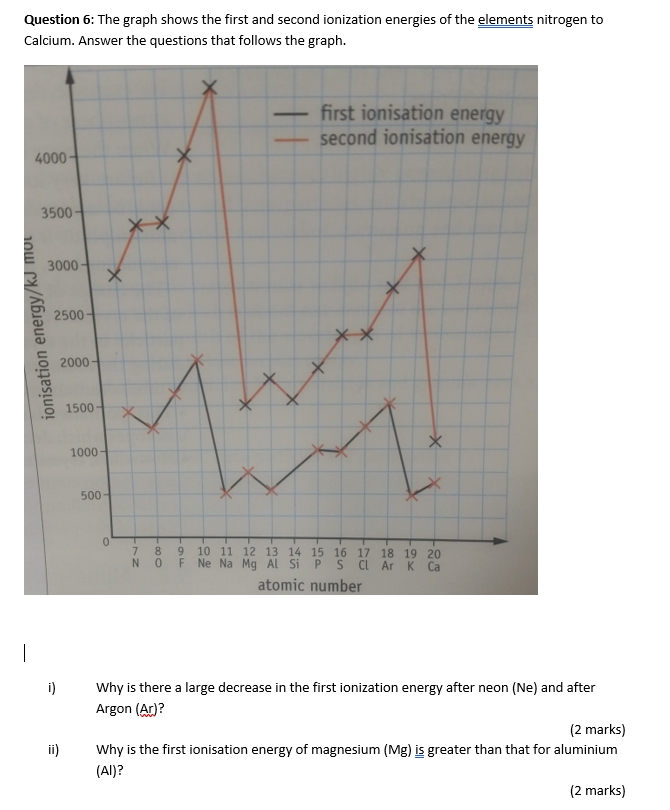


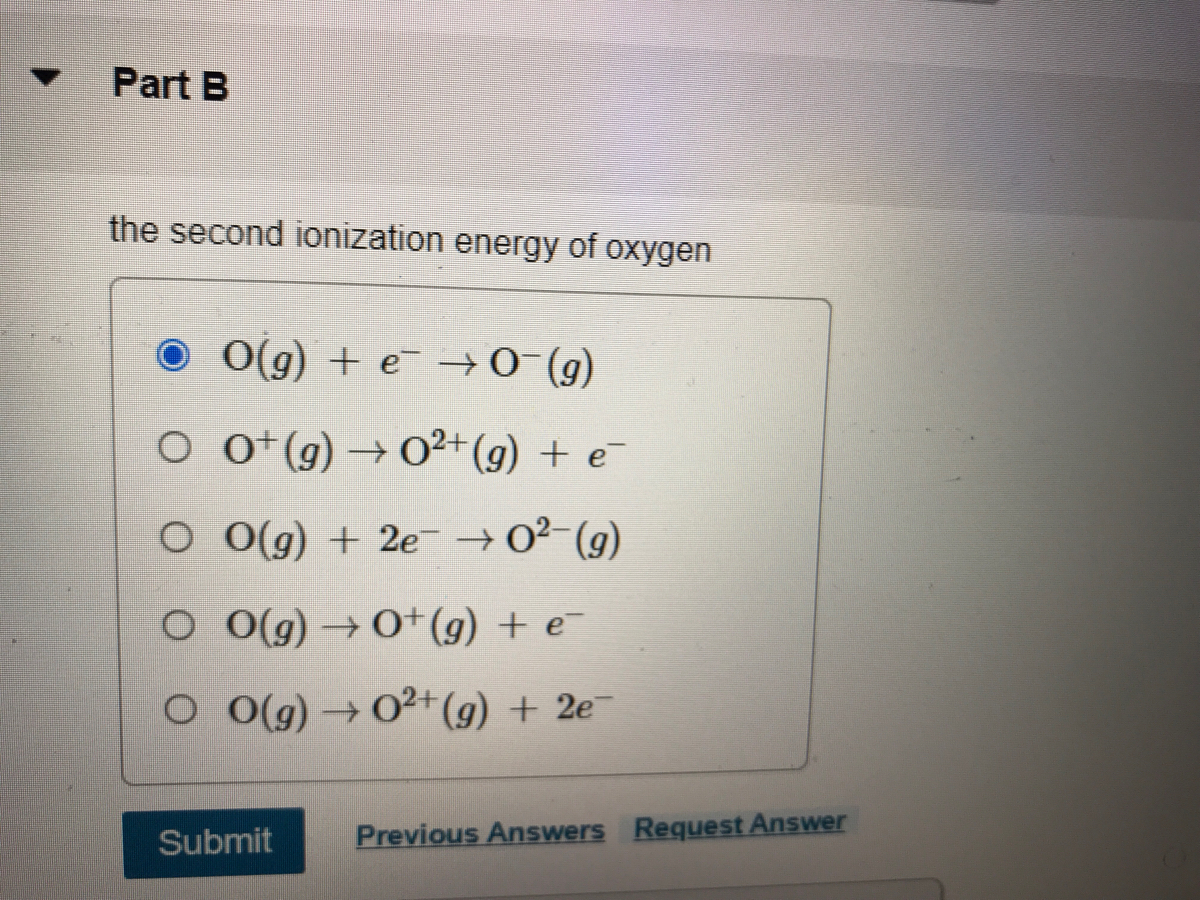
![Q. Solved] Which Ion Was Formed By Providing The Second Ionization Energy To Remove An Electron? Q. Solved] Which Ion Was Formed By Providing The Second Ionization Energy To Remove An Electron?](https://1.bp.blogspot.com/-WB3uY9wfNBo/X8aaJmP4A8I/AAAAAAAAAdk/bCT6kOXT44gjbnsCqNG7dfgwfcQ7eA8DgCPcBGAYYCw/s1918/Periodic%2BTable.jpg)