AzurRx BioPharma and Mayoly Spindler Announce MS1819-SD Investigational Medicinal Product Dossier (IMPD) Submission | BioSpace

How to achieve safe, high-quality clinical studies with non-Medicinal Investigational Products? A practical guideline by using intra-bronchial carbon nanoparticles as case study | Respiratory Research | Full Text
How to achieve safe, high-quality clinical studies with non-Medicinal Investigational Products? A practical guideline by using i

Sharp Clinical Services exhibiting at Clinical Trial Logistics in Brussels | Press Release Distribution

Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products (NIMPs) - PDF Free Download
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials
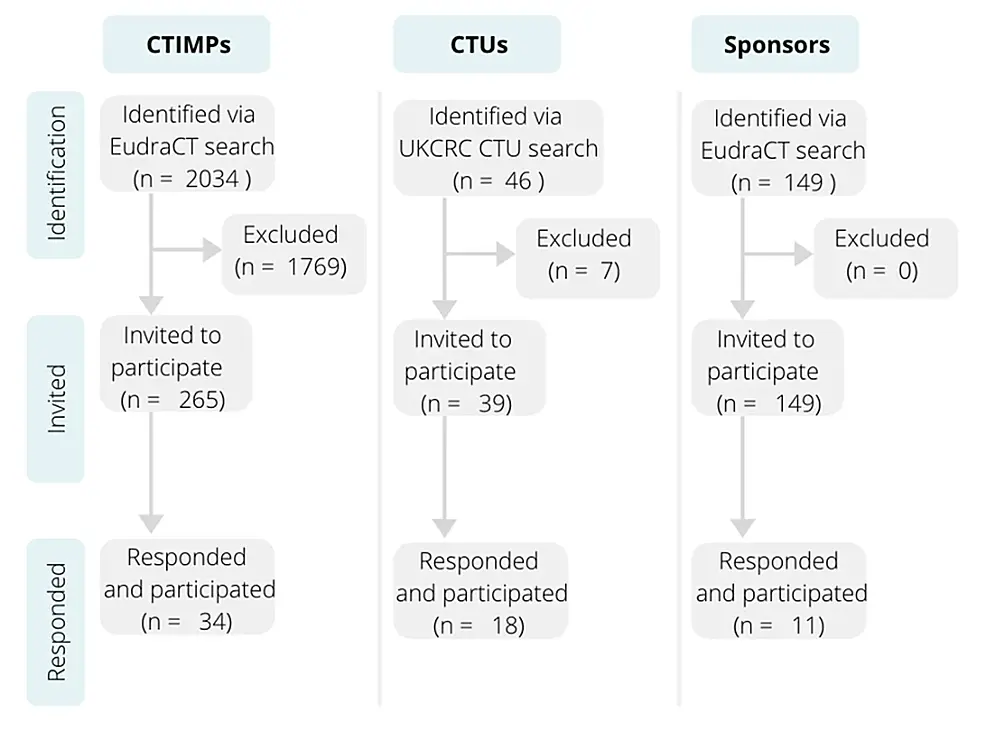
Cureus | A Snapshot of the Response from UK-based Clinical Trials of Investigational Medicinal Products to COVID-19 | Article