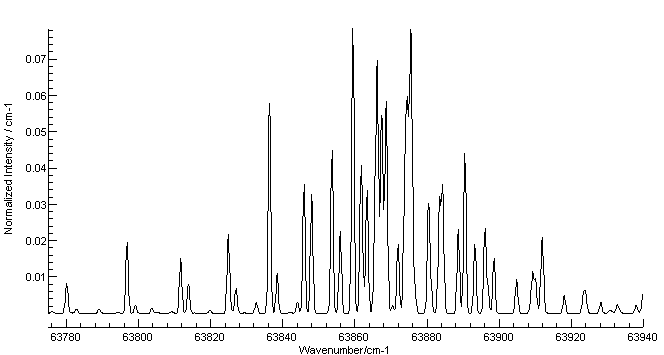
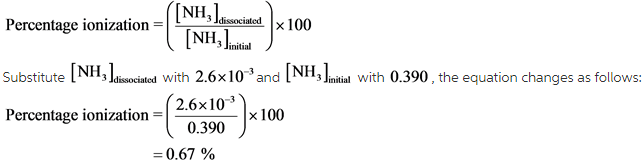Energy spectrum of the electrons emitted in the ionization of ammonia... | Download Scientific Diagram

Quantitative Operando Detection of Electro Synthesized Ammonia Using Mass Spectrometry - Krempl - 2022 - ChemElectroChem - Wiley Online Library
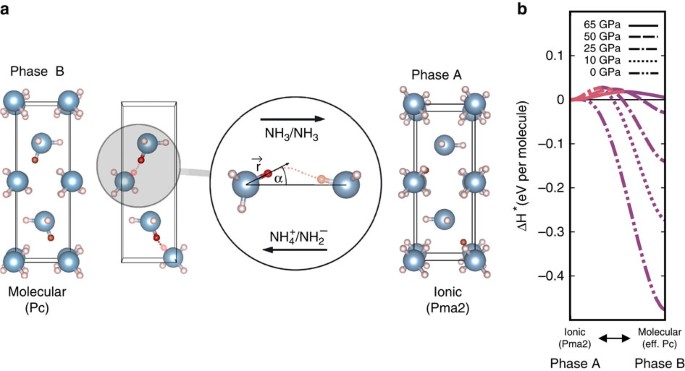
Ammonia as a case study for the spontaneous ionization of a simple hydrogen-bonded compound | Nature Communications

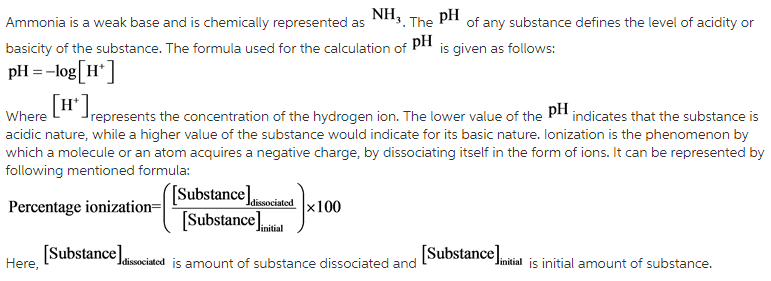
Liquid ammonia ionises to a slight extent. At `-50^(@)C`, its self ionisation constant, `K_(NH_(3))= - YouTube
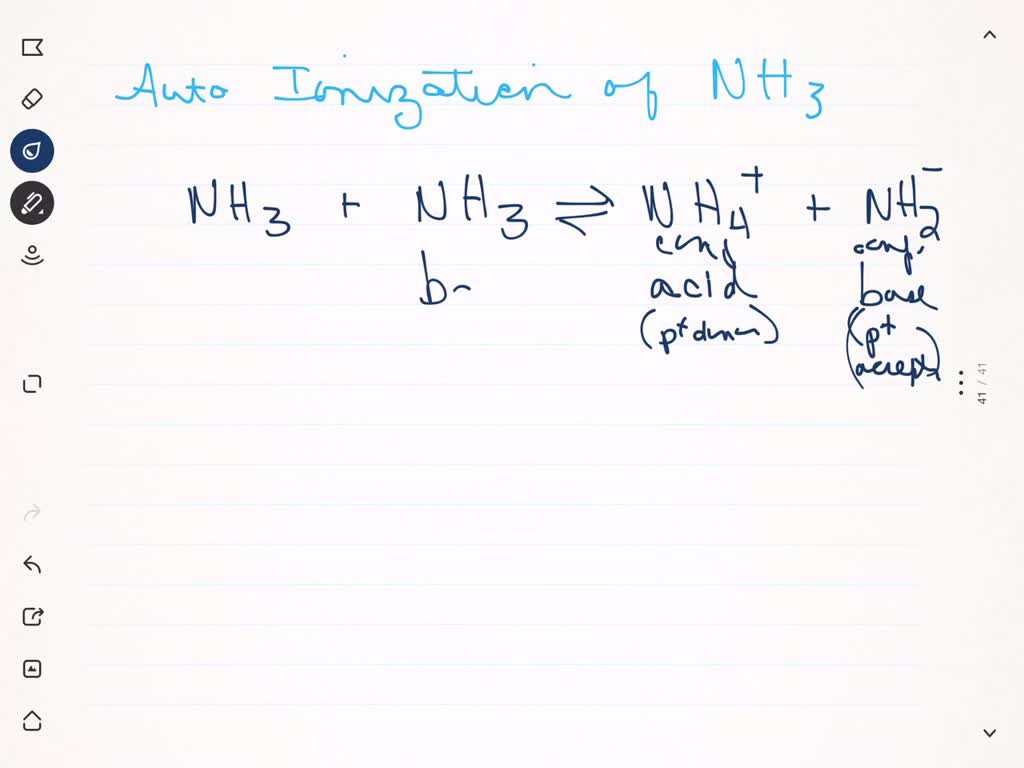
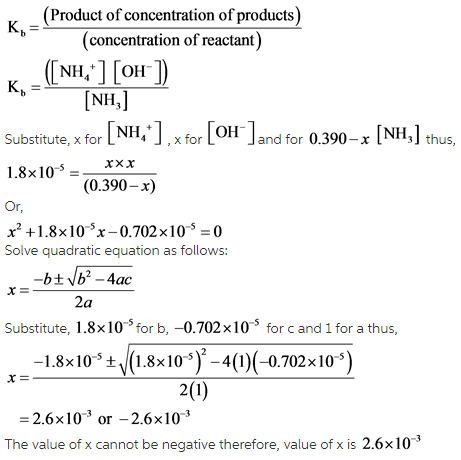
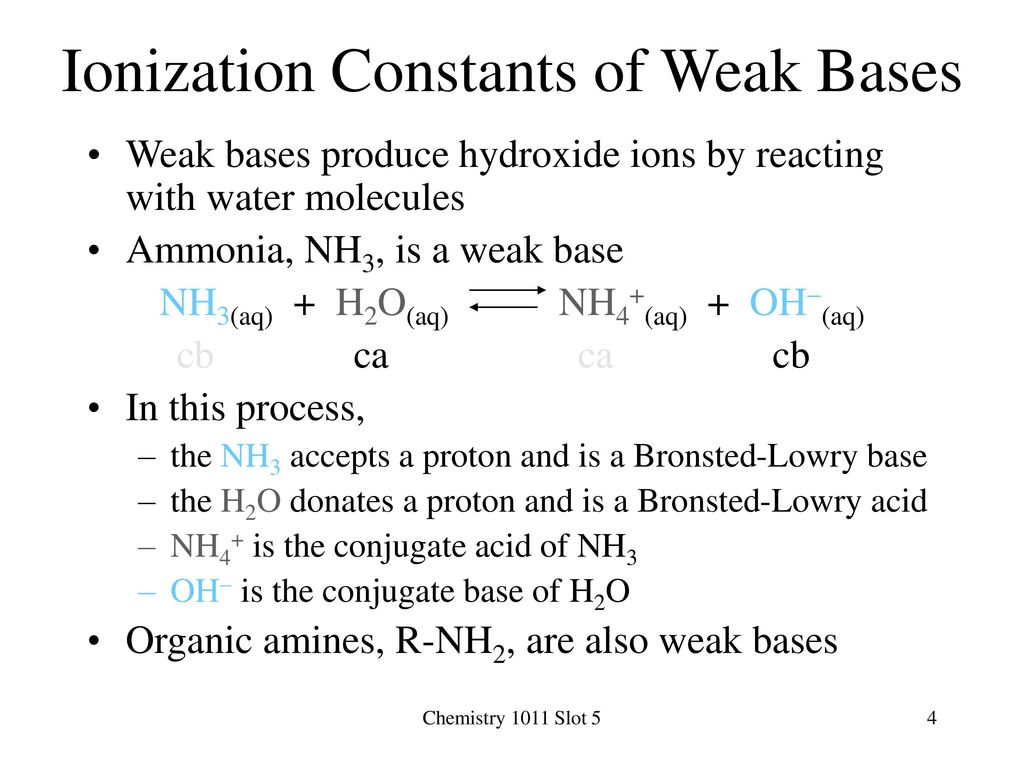
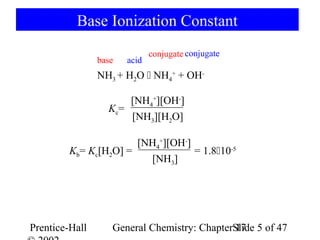
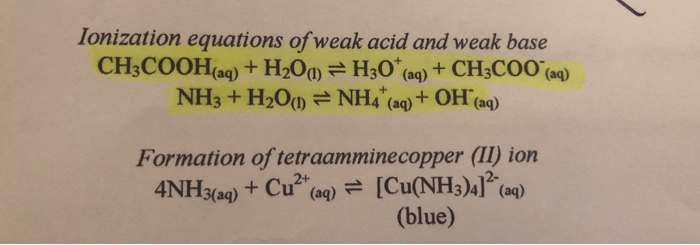
SOLVED:Like water, liquid ammonia undergoes autoionization: NH3+NH3 ⇌NH4^++NH2^- (a) Identify the Bronsted acids and Bronsted bases in this reaction. (b) What species correspond to H^+ and OH^- and what is the condition

Energy spectrum of the electrons emitted in the ionization of ammonia... | Download Scientific Diagram
![At - 50^(@) C, the self - ionization constant (ion product ) of NH(3)" is " K(NH(3)) = [NH(4)^(+)] [NH(2)^(-)] = 10^(-30) M^(2). How many amide ions are present per mm^(3) of pure liquid ammonia ? At - 50^(@) C, the self - ionization constant (ion product ) of NH(3)" is " K(NH(3)) = [NH(4)^(+)] [NH(2)^(-)] = 10^(-30) M^(2). How many amide ions are present per mm^(3) of pure liquid ammonia ?](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/261016062_web.png)
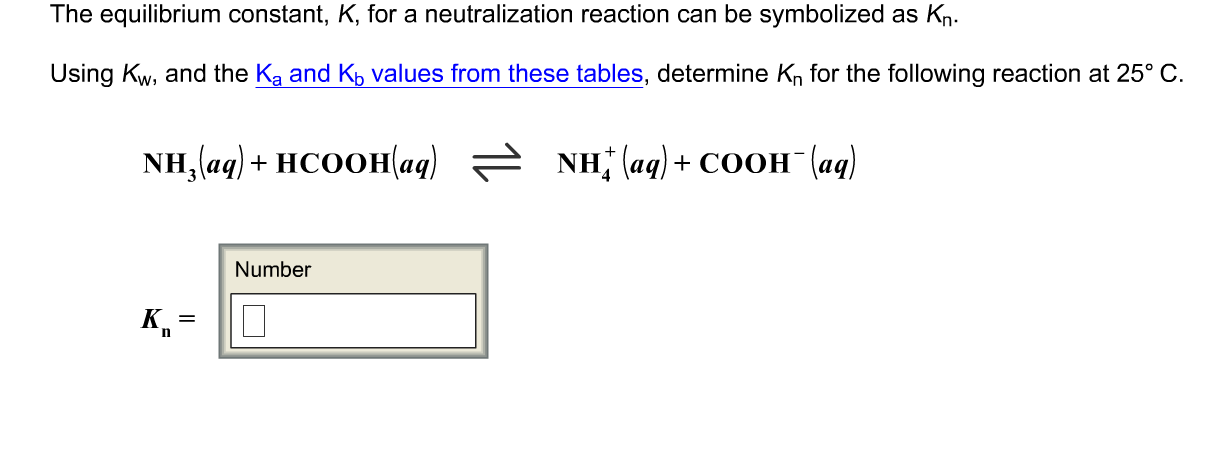
At - 50^(@) C, the self - ionization constant (ion product ) of NH(3)" is " K(NH(3)) = [NH(4)^(+)] [NH(2)^(-)] = 10^(-30) M^(2). How many amide ions are present per mm^(3) of pure liquid ammonia ?
![Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 ) Liquid ammonia ionizes to slight extent. At - 50^∘C , its self - ionization constant, K = [NH4^+] [NH2^-] = 10^-30 M^2 . How many amide ions are present per ml of pure liquid ammonia? ( NA = 6 × 10^23 )](https://dwes9vv9u0550.cloudfront.net/images/11548439/2313bf3e-ac46-4626-8ef0-7c7a53c9fba2.jpg)