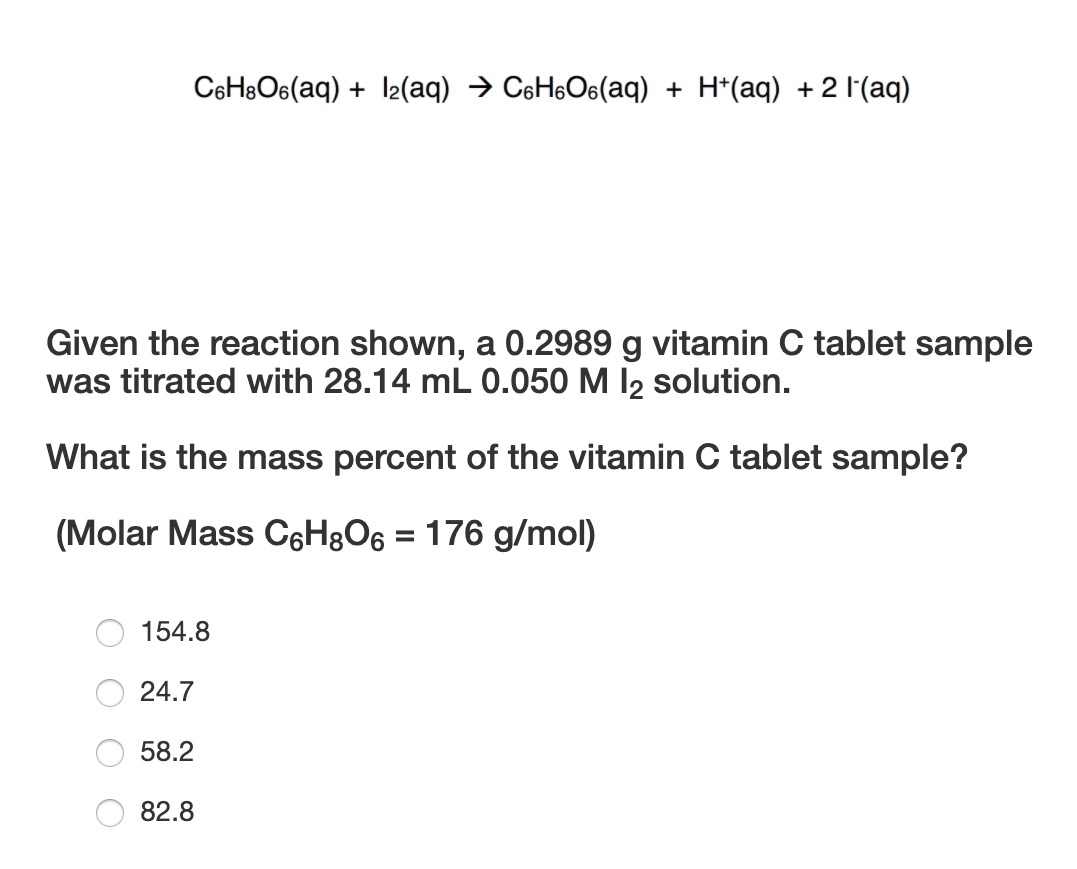
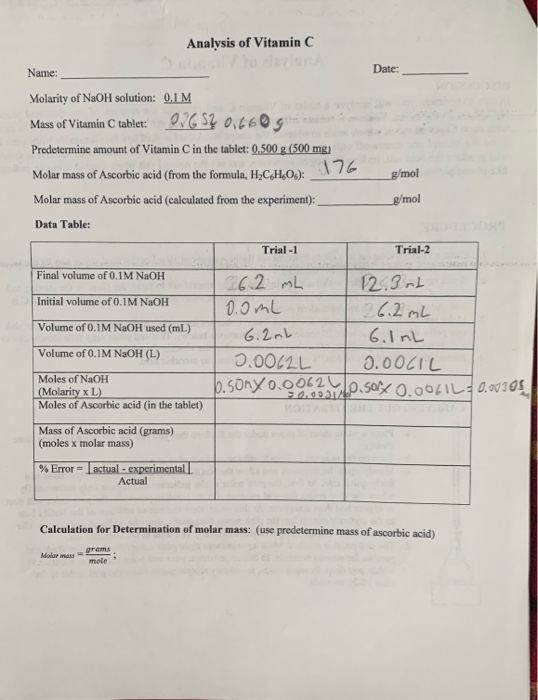
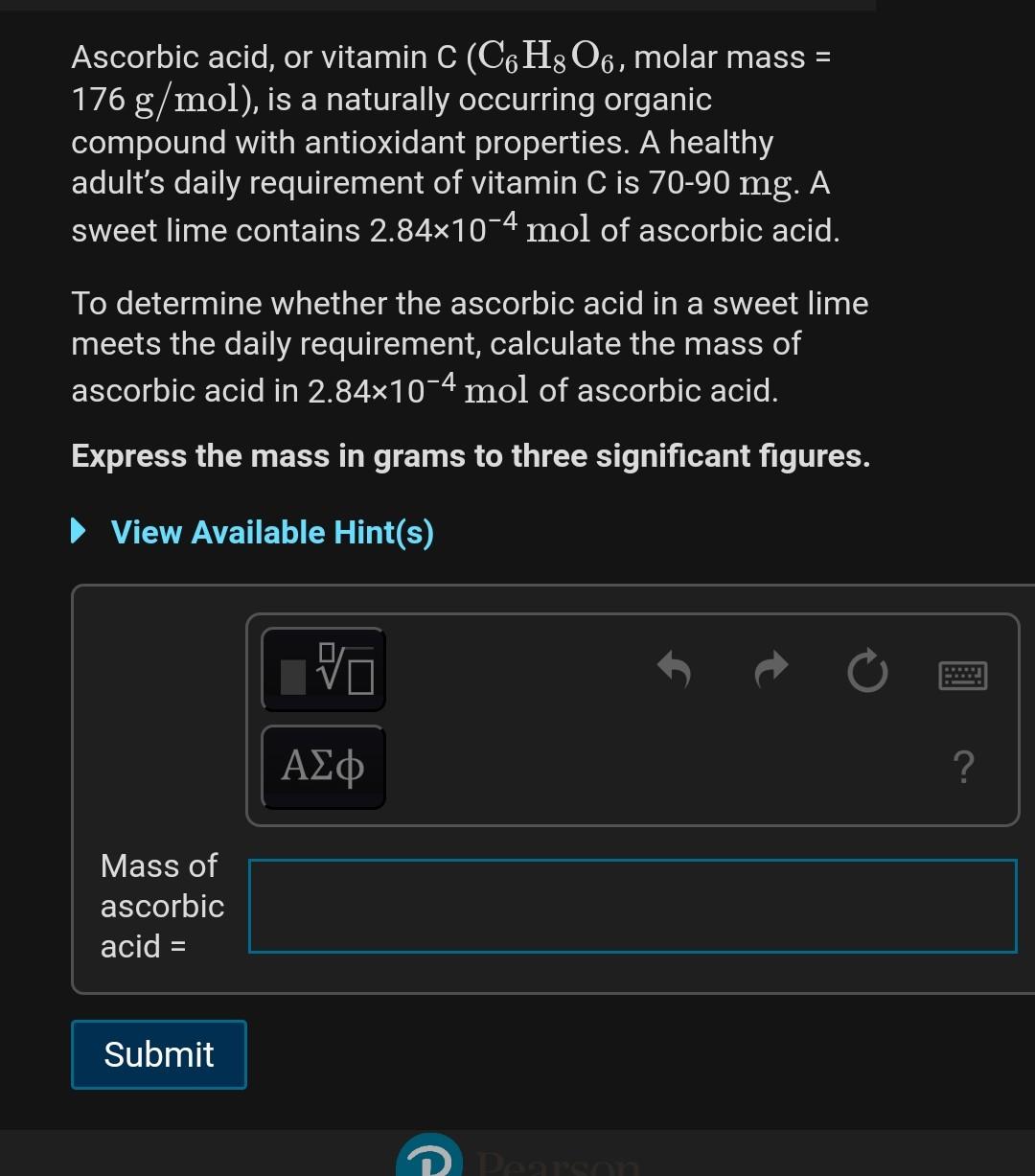
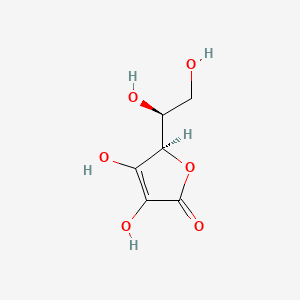
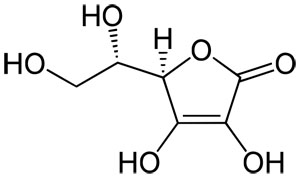
SOLVED: The molar mass of ascorbic acid (vitamin C) is 176.12 g/mol. Ascorbic acid contains C, H, and 0. Mass percent composition analysis shows that this compound contains 40.92% C, 4.58% H

Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolved in 75g of acetic acid to.... - YouTube

Calculate the mass of ascorbic acid (C6H8O6) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5^0C . (Kf for acetic acid is 3.9 K kg mol^-1 ).
Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) - Sarthaks eConnect | Largest Online Education Community

Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5^@C. Kf = 3.9 K kg "mol"^(-1) .
![Solved] Calculate the mass of ascorbic acid to be dissolved in 75g of acetic acid to lower its - Brainly.in Solved] Calculate the mass of ascorbic acid to be dissolved in 75g of acetic acid to lower its - Brainly.in](https://hi-static.z-dn.net/files/dcf/9c7caa88d0eb3f9e2f4686f7fccfbdb2.jpg)
Solved] Calculate the mass of ascorbic acid to be dissolved in 75g of acetic acid to lower its - Brainly.in

SOLVED: How many molecules of Vitamin C are in 500. mg of Vitamin € ( Molar mass of vitamin € = 176 g/mol)? 6.022*1023 1.71 X1023 1.71 x10 24 1.71 x1021

SOLVED: The molecular formula of ascorbic acid (vitamin C) is C6H8O6. Determine the percent composition by mass in vitamin C (the percent of each constituent element).

Calculate the mass of ascorbic acid (Vitamin C. C6H8O6 ) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5^∘C. Kf = 3.9K kg mol^- 1 .

Calculate the mass of ascorbic acid (Vitamin C. C6H8O6 ) to be dissolved in 75 g of acetic acid to lower its melting point by 1.5^∘C. Kf = 3.9K kg mol^- 1 .

Calculate the mass of ascorbic acid ( Vitamin C,C6H8O6 ) to be disssolved in 75 g of acetic acid to lower its melting point by 1.5.Kf = 3.9 K ^-1 .
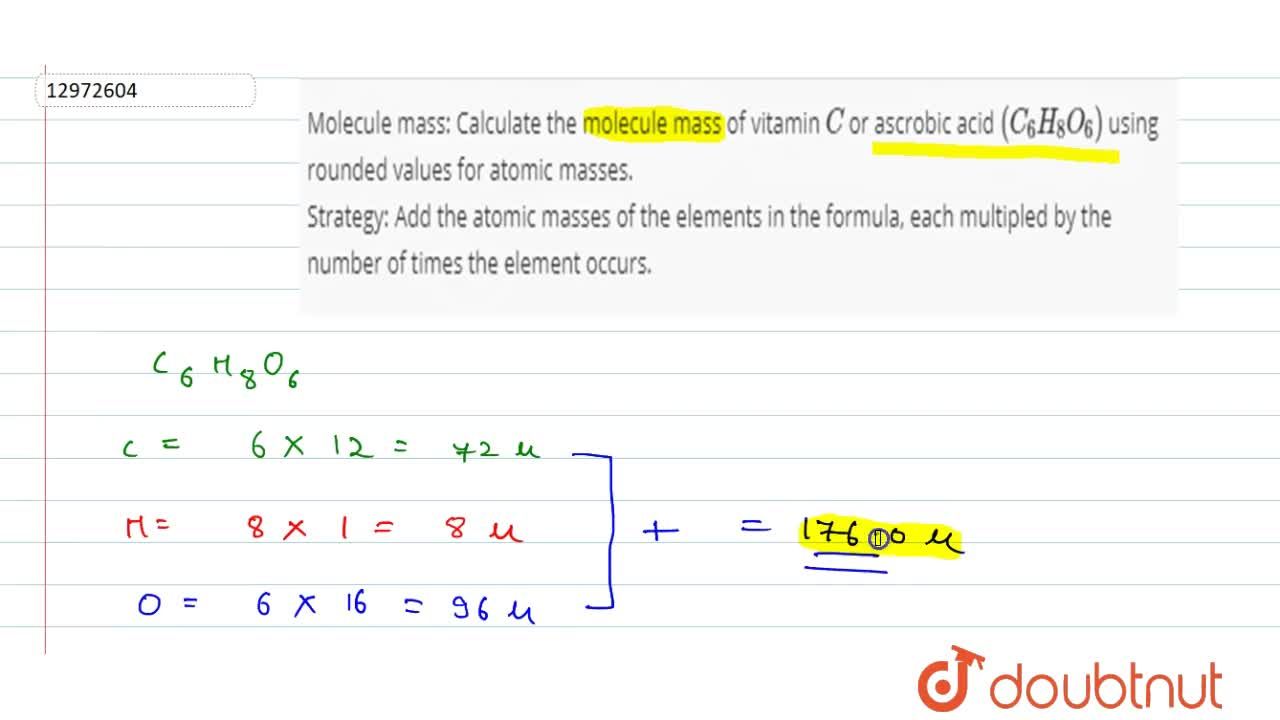
Molecule mass: Calculate the molecule mass of vitamin C or ascrobic acid (C(6) H(8) O(6)) using rounded values for atomic masses. Strategy: Add the atomic masses of the elements in the formula,