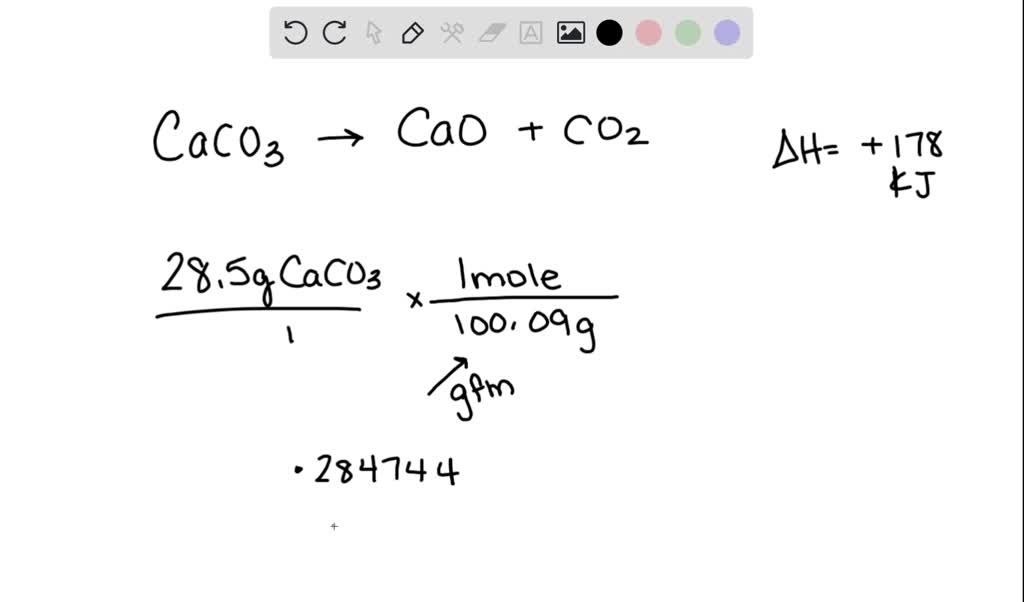
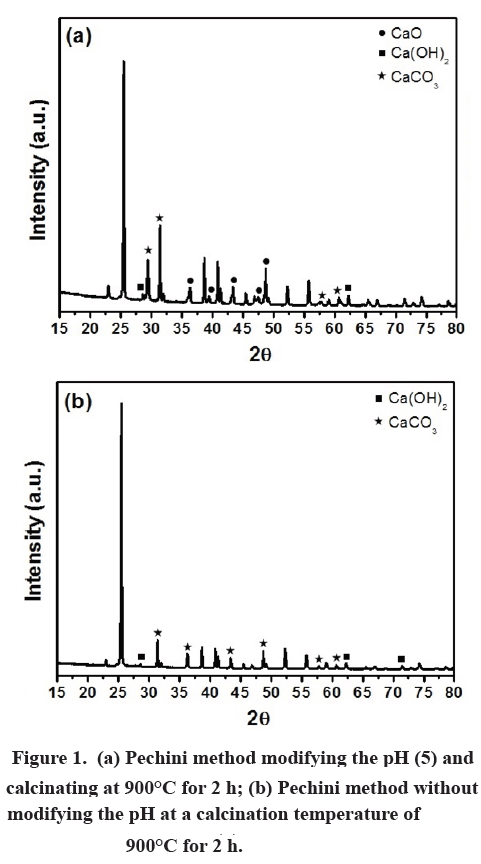
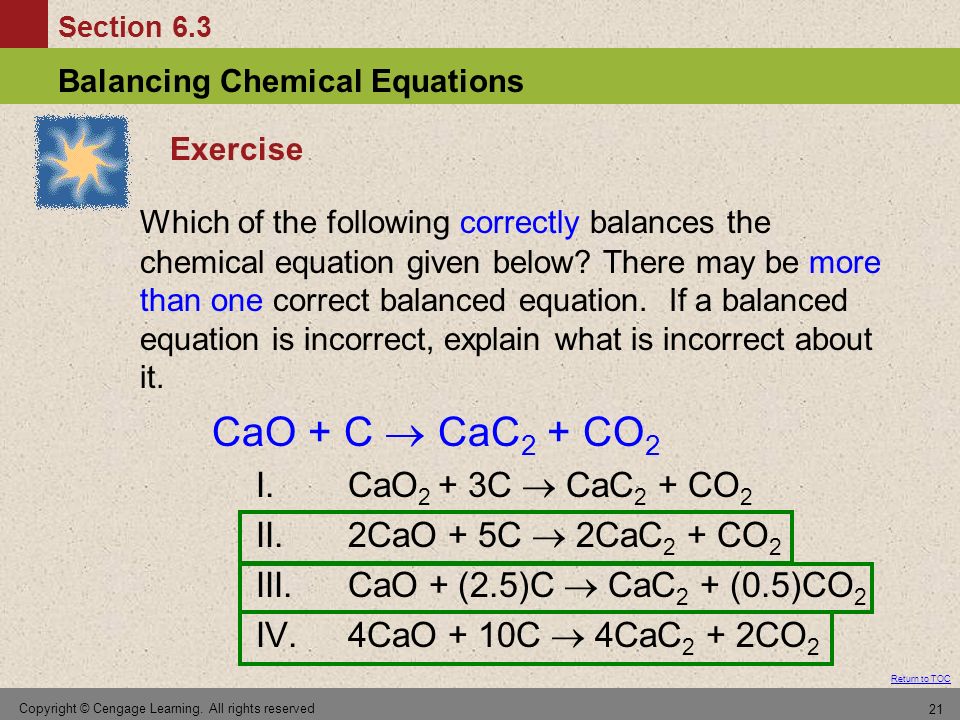
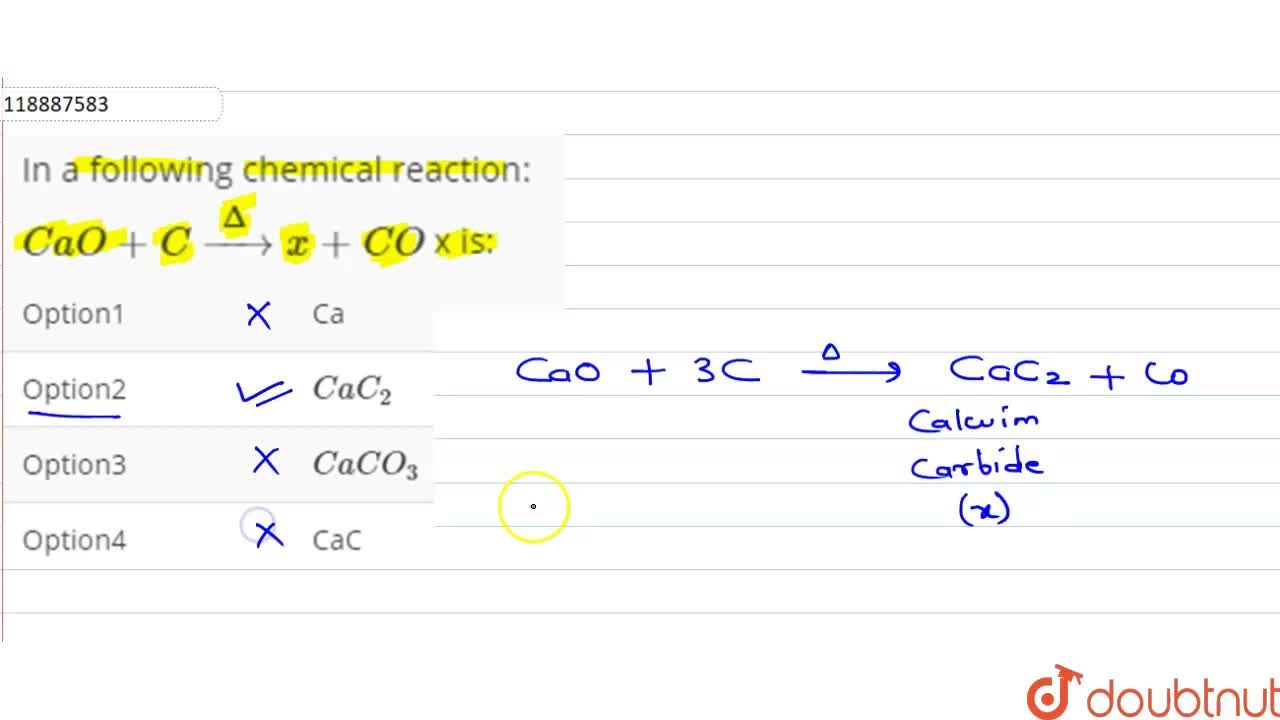
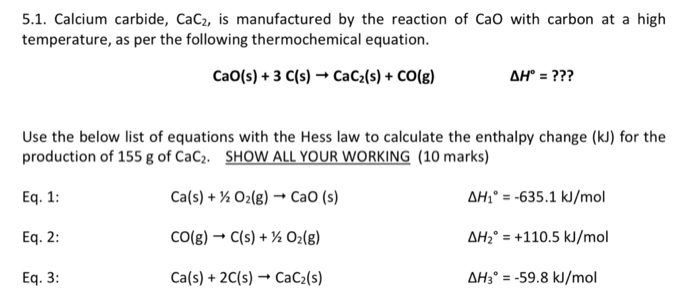
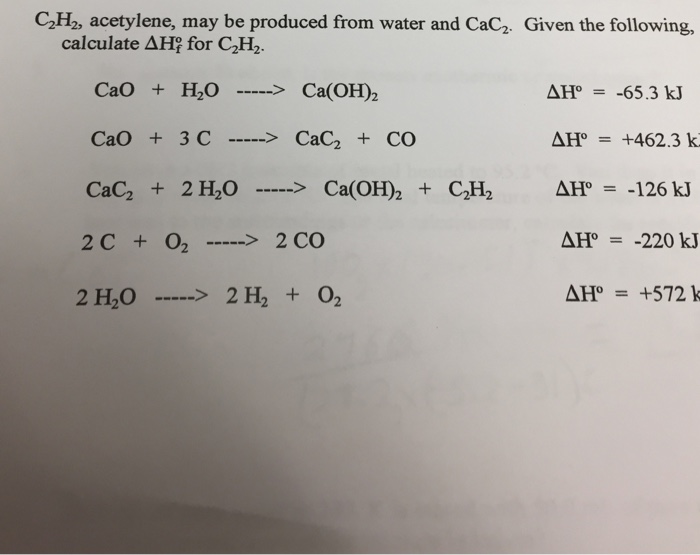H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C - ScienceDirect

i) CaO(s) + H2O (l) = Ca (OH)2(s) , Δ H180^oC = - 15.26 kcal (ii) H2O(l) = H2(g) + 12 O2 (g) , Δ H180^oC = 68.37 kcal (iii) Ca(s) +

Chemical Equation A representation of a chemical reaction: C 2 H 5 OH + 3O 2 2CO 2 + 3H 2 O reactants products. - ppt download

Chemical Reactions Follow the matter… 1. Chemical Reactions Two chemicals have interacted in some way so that a new substance or substances are formed. - ppt download

CO2 Adsorption on CaO(001): Temperature-Programmed Desorption and Infrared Study | The Journal of Physical Chemistry C

Autothermal CaO Looping Biomass Gasification for Renewable Syngas Production | Environmental Science & Technology

Phase diagram of FexO-SiO2-CaO-MgO-"NiO"system, at PO2 = 10 −7 atm,... | Download Scientific Diagram