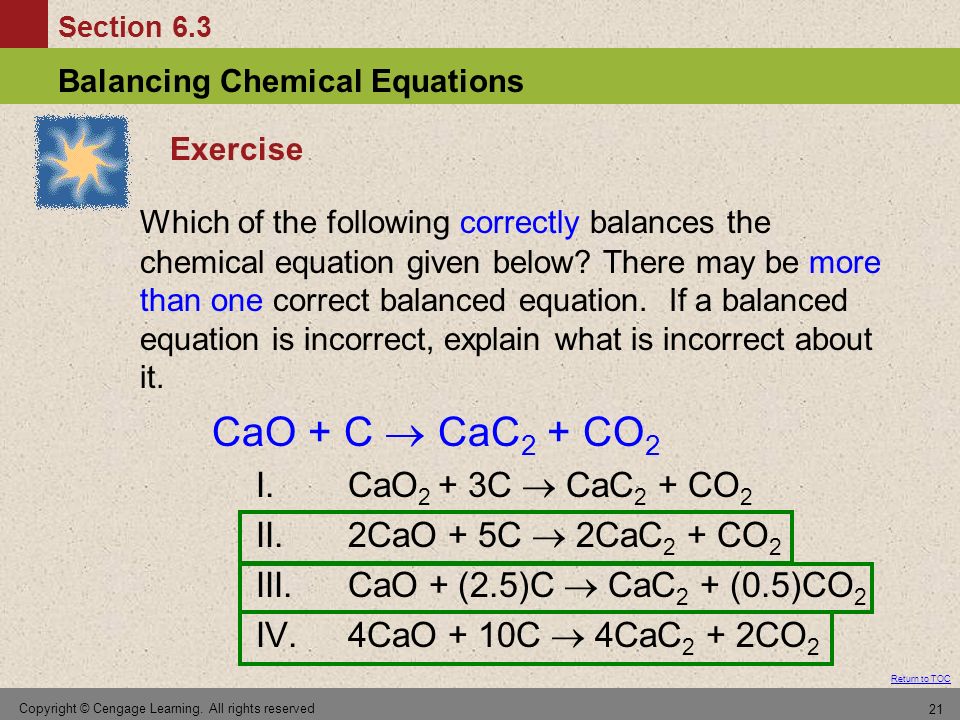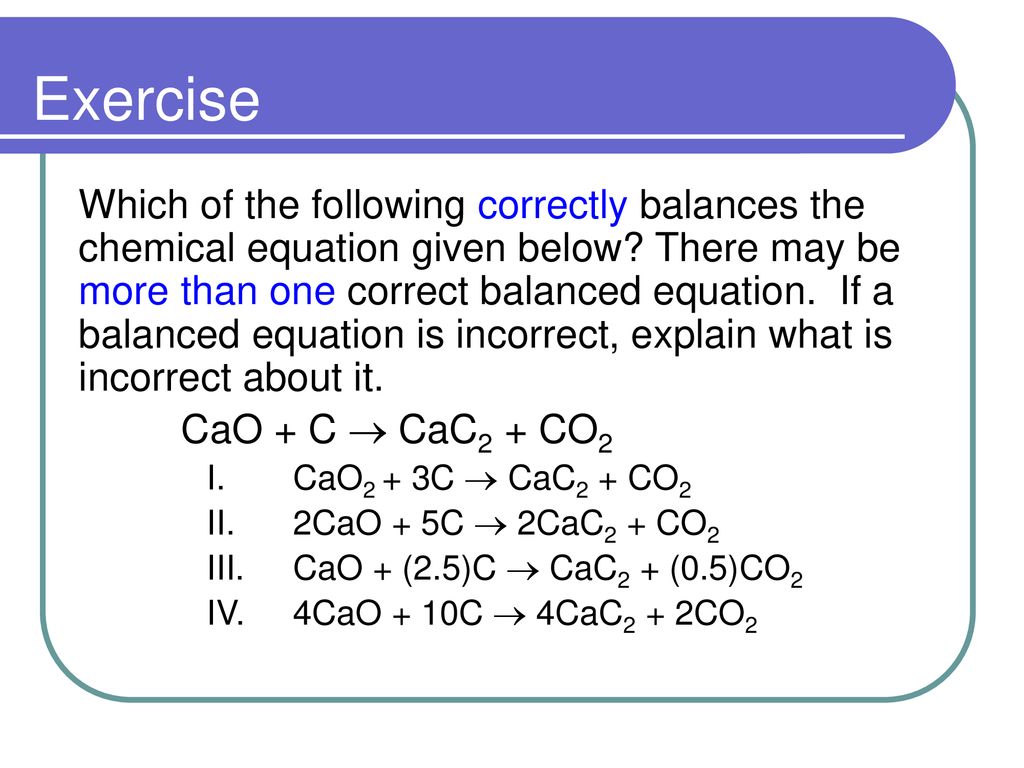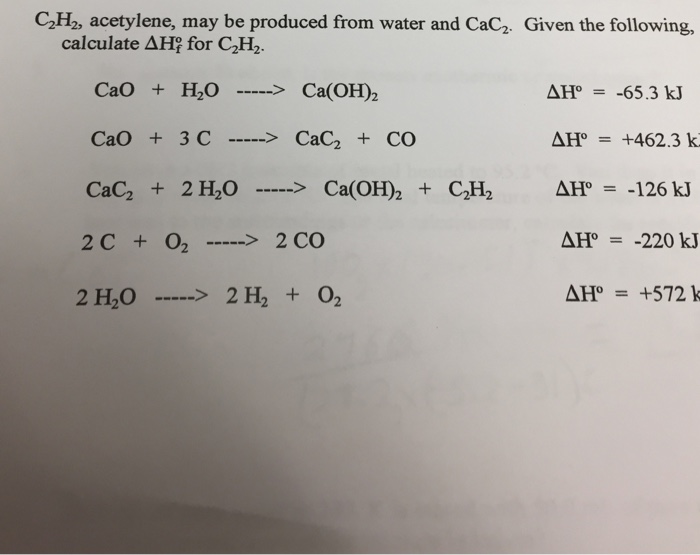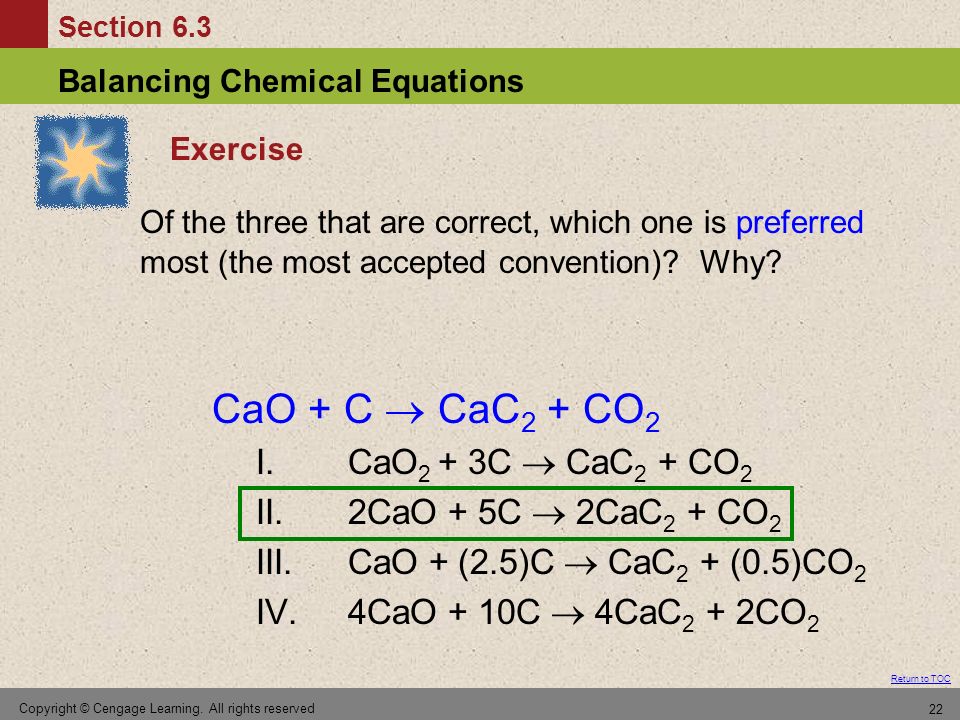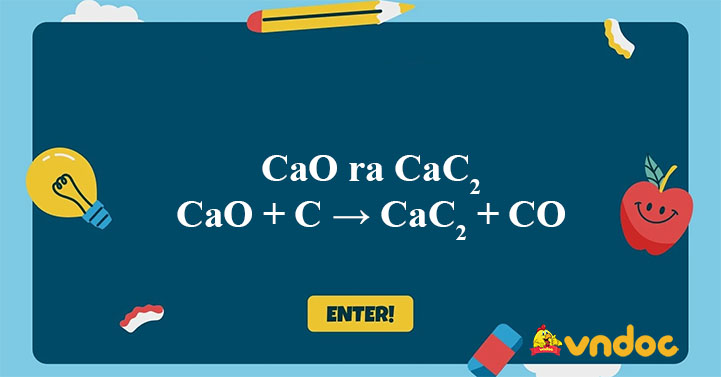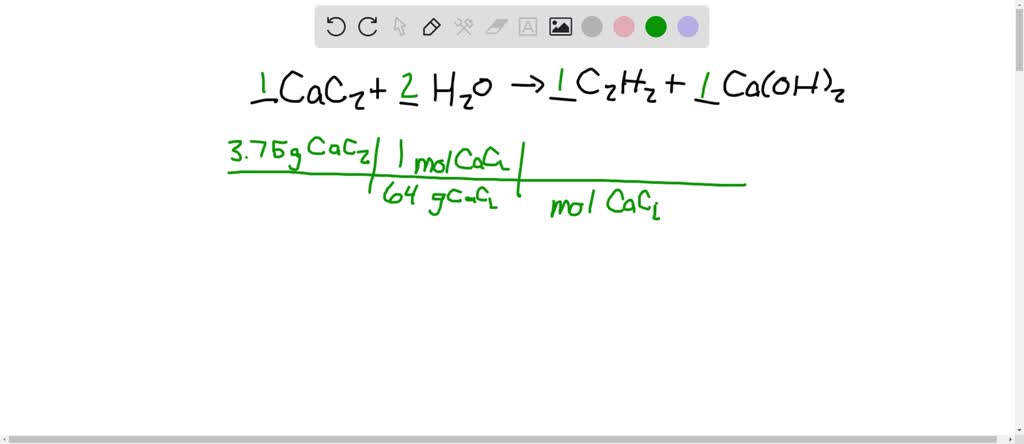
SOLVED:Calcium carbide, CaC2, can be produced in an electric furnace by strongly heating calcium oxide (lime) with carbon. The unbalanced cquation is CaO(s)+C(s) →CaC2(s)+CO(g) Calcium carbide is useful because it reacts readily

Calcium Carbide: A Unique Reagent for Organic Synthesis and Nanotechnology - Rodygin - 2016 - Chemistry – An Asian Journal - Wiley Online Library

Assign the sign of work done (based on SI Convention) in the following chemical changes taking place against external atmospheric pressure:I. N2 (g) + 3H2 (g) ⟶ 2NH3 (g) II. NH4HS (s)
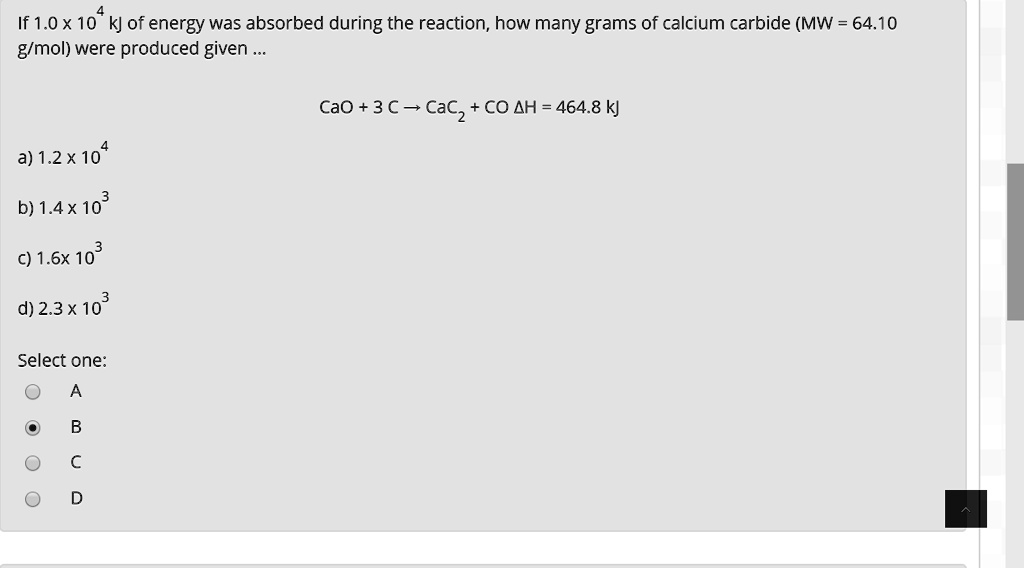
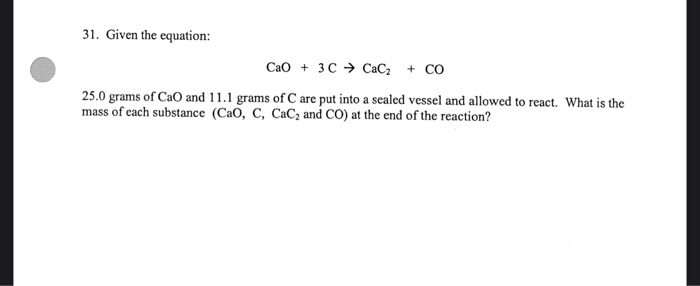
SOLVED: If 1.0X 10 kJ of energy was absorbed during the reaction, how many grams of calcium carbide (MW = 64.10 glmol) were produced given Cao + 3 € CaC2 + CO
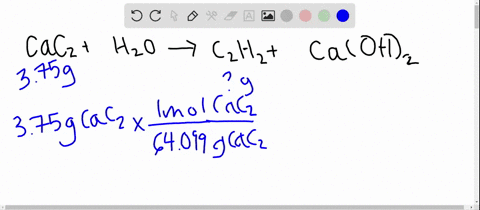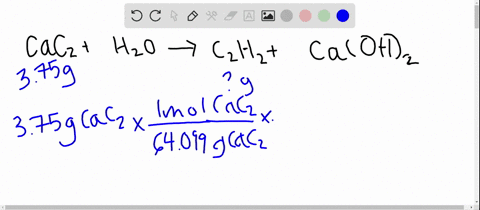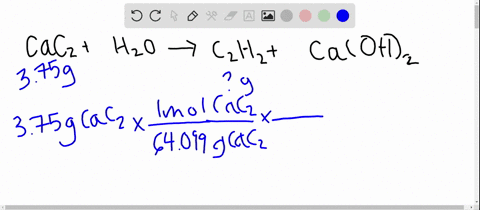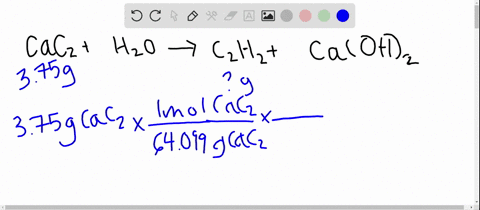
SOLVED: Calcium carbide, CaC2, used to produce acetylene, C2H2, is prepared by heating calcium oxide, CaO, and carbon, C, to high temperature. CaO(s)+ C(s) Cac2(s)+ CO (g) Balance the chemical equation for
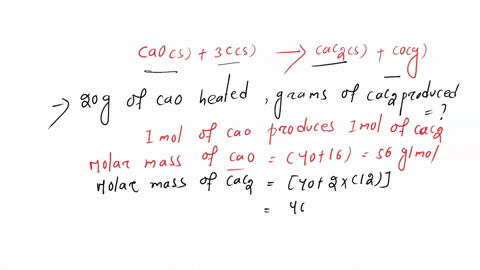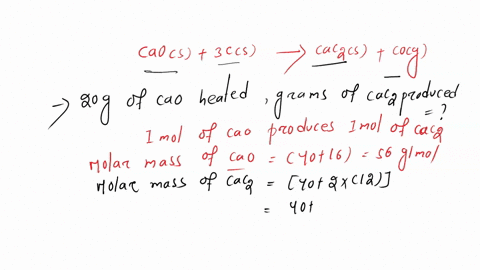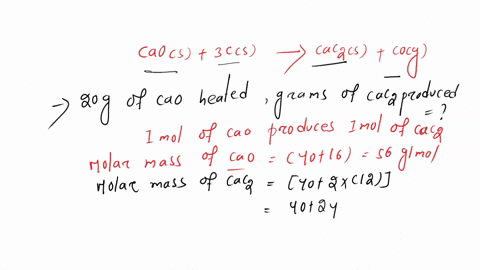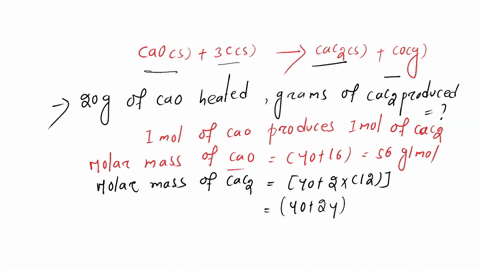
SOLVED: Calcium carbide, CaC2, can be prepared at high temperature by the following reaction: CaO(s) + C(s) -> CaC2(s) + CO(g) a) Balance the chemical equation. b) If a starting mixture contains
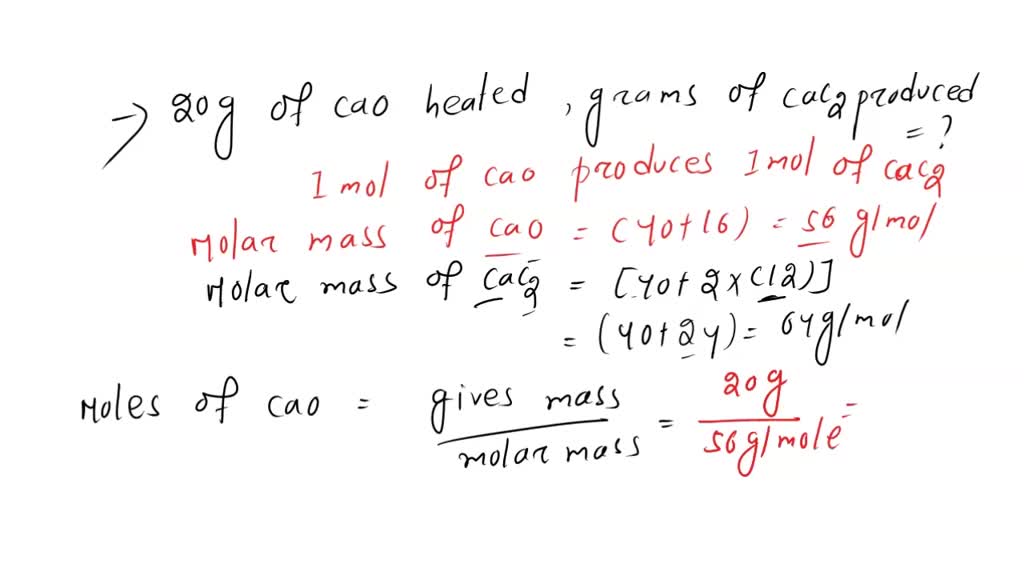
SOLVED: Calcium carbide, CaC2, used to produce acetylene, C2H2, is prepared by heating calcium oxide, CaO, and carbon, C, to high temperature. CaO(s)+ C(s) Cac2(s)+ CO (g) Balance the chemical equation for
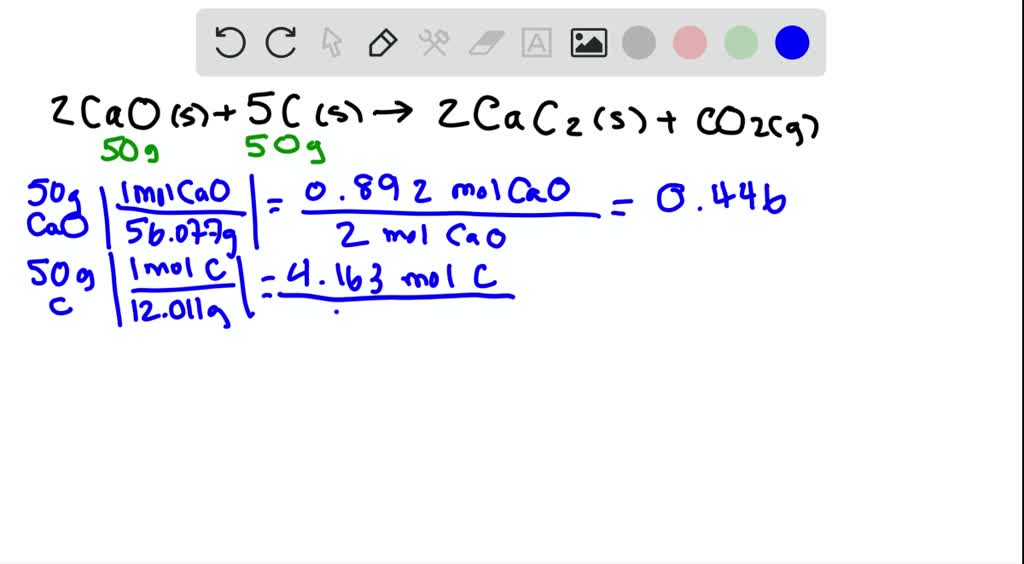
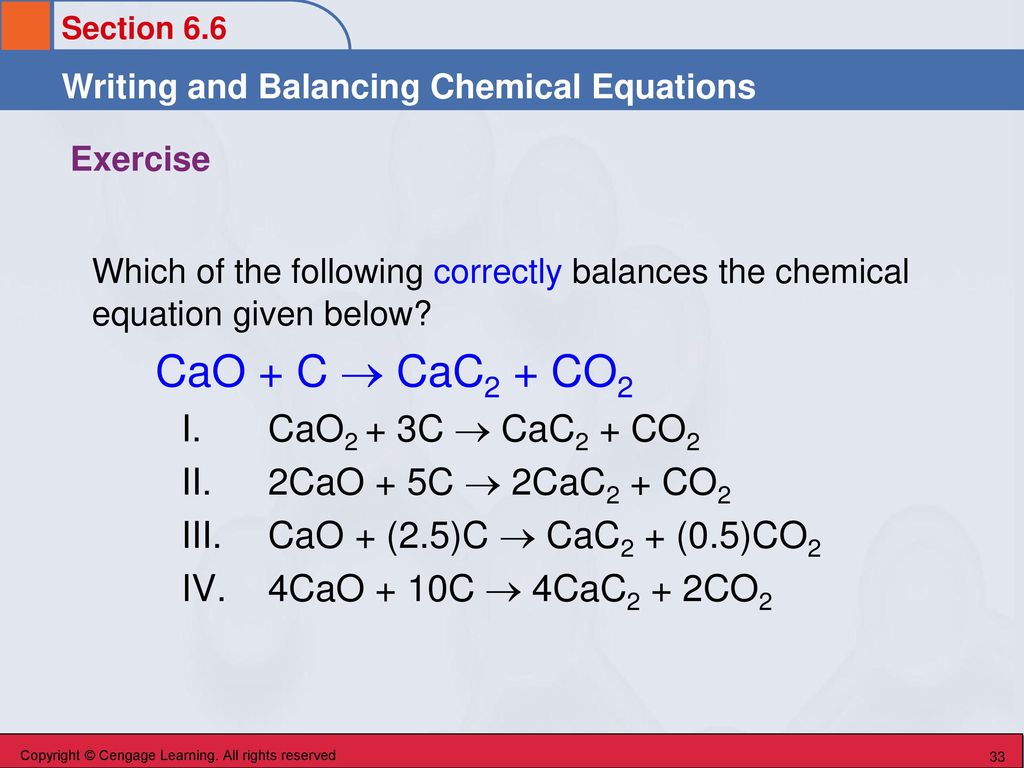
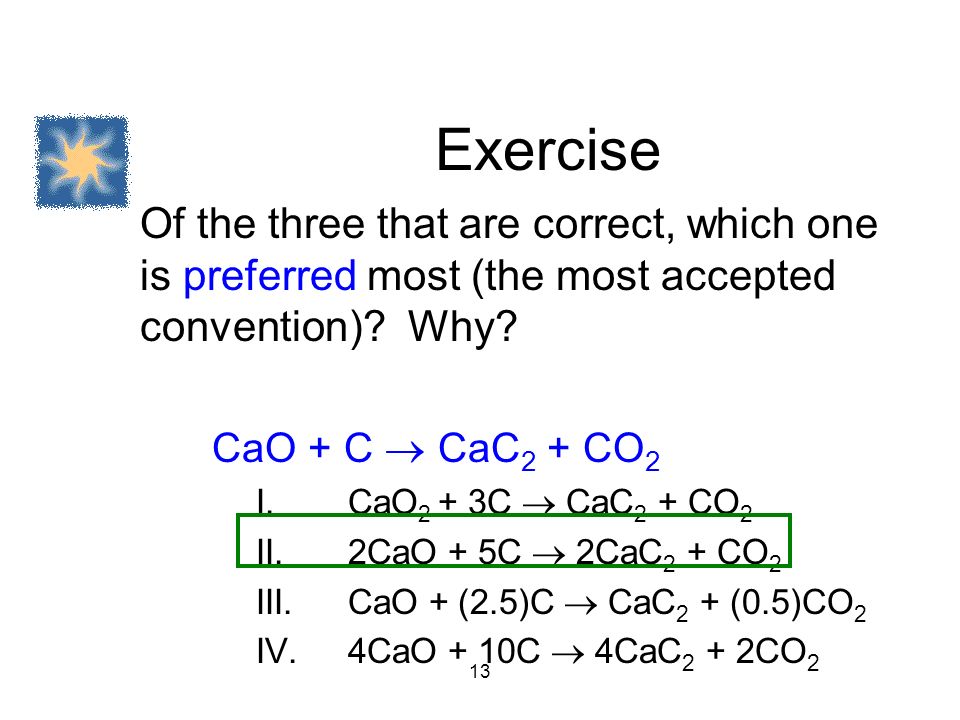
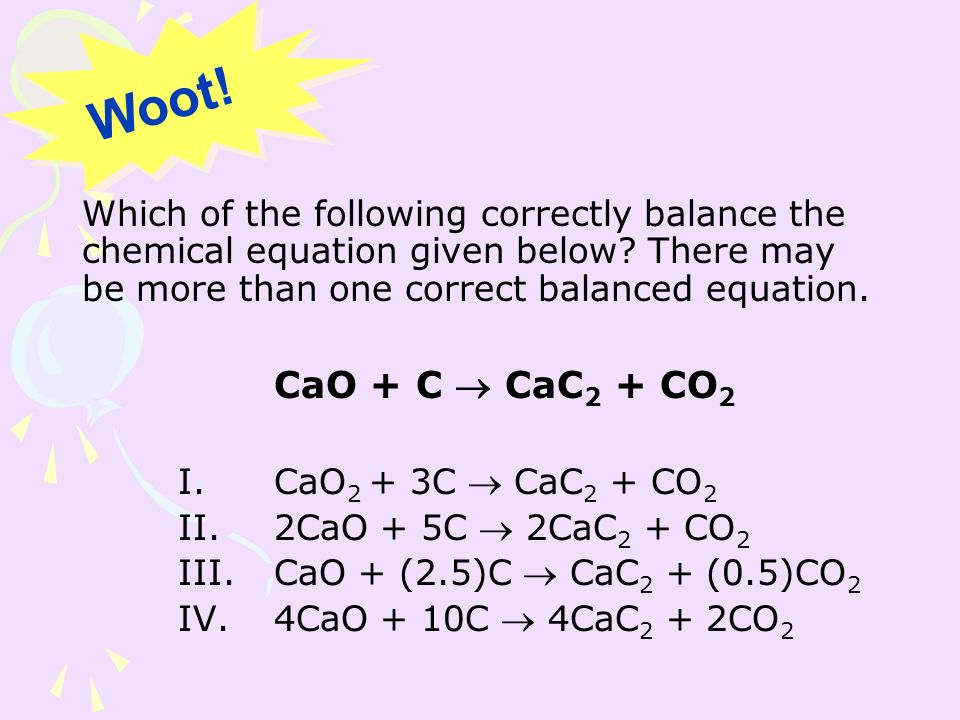
SOLVED:Determine the limiting reactant and mass of left-over reactant when a 50.0 g sample of CaO is reacted with 50.0 g of carbon according to the balanced equation 2 CaO(s)+5 C(s) →2
Chemical Reactions Follow the matter… 1. Chemical Reactions Two chemicals have interacted in some way so that a new substance or substances are formed. - ppt download
Chemical Equation A representation of a chemical reaction: C 2 H 5 OH + 3O 2 2CO 2 + 3H 2 O reactants products. - ppt download

From the following reactions at 298 K .(A) CaC2(s) + 2H2O(l) → Ca(OH)2(s) + C2H2 (g); Δ H^∘ = - 127.9 kJ mol^-1 (B) Ca(s) + 12 O2(g) → CaO(s) ; Δ