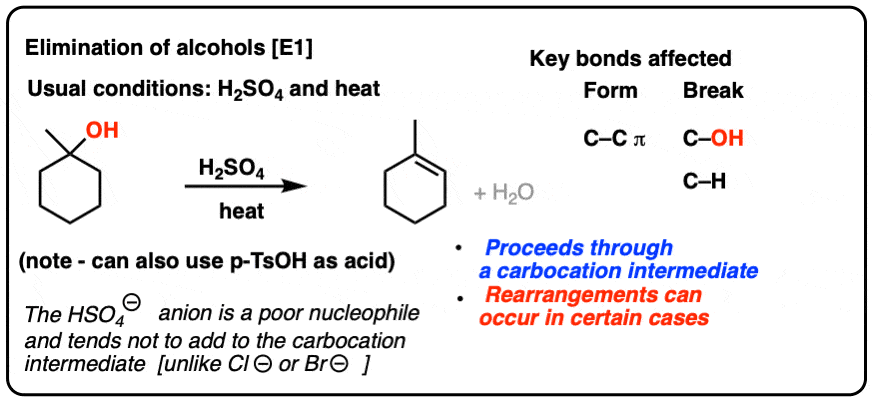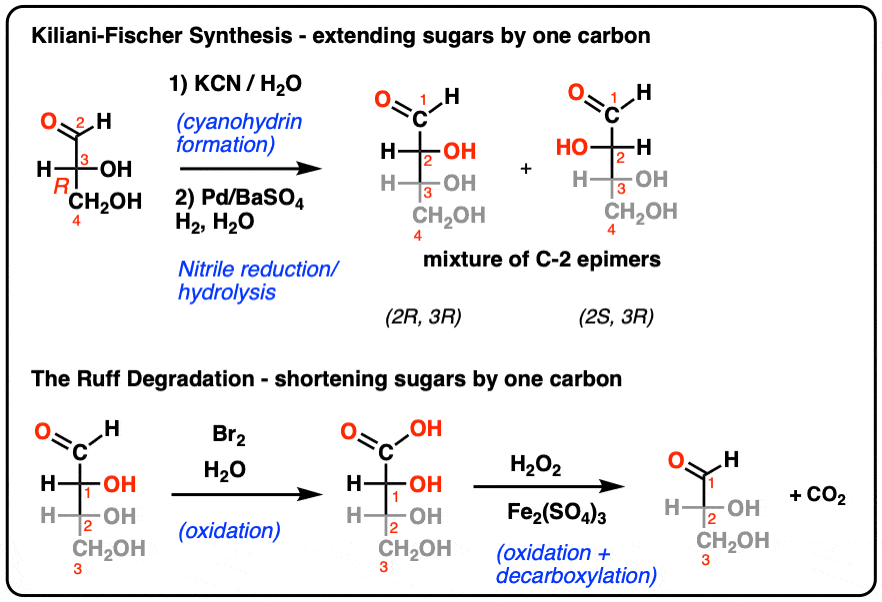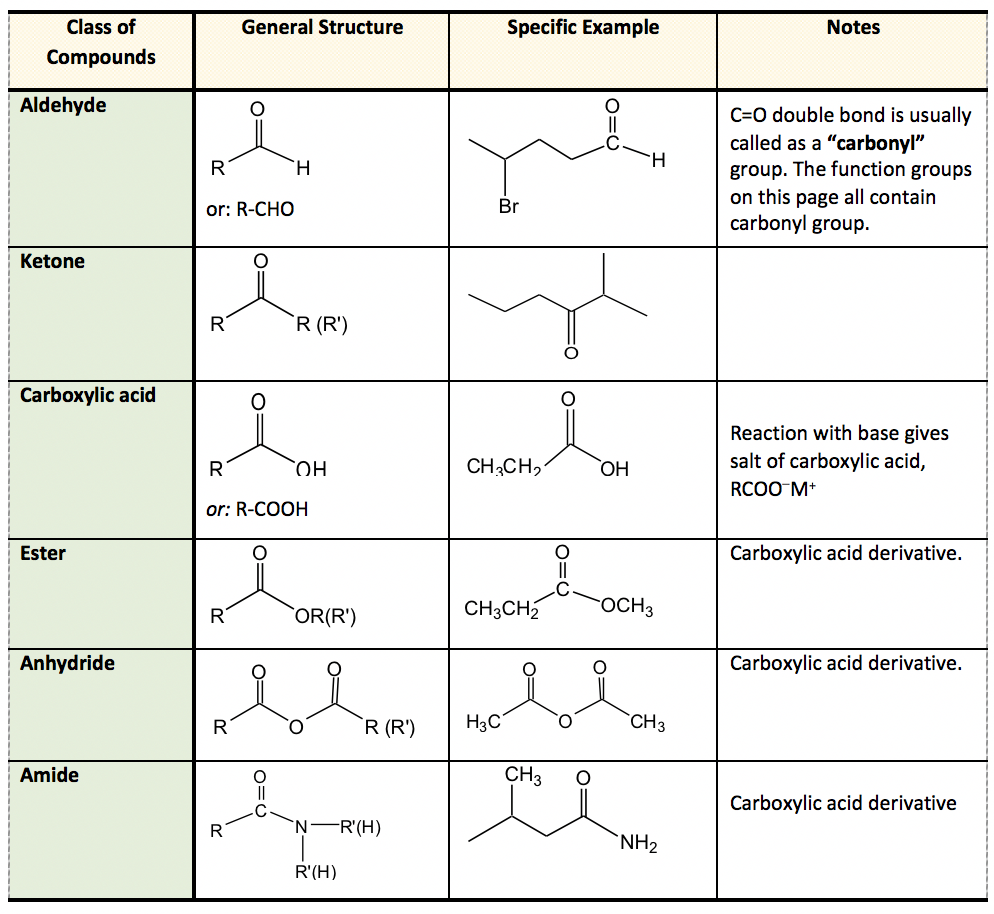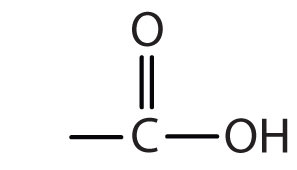
Chapter 2 - Alcohols, Phenols, Thiols, Ethers - CHE 120 - Introduction to Organic Chemistry - Textbook - LibGuides at Hostos Community College Library

How to name alcohols alkanols ethers naming structure nomenclature isomers general empirical structural skeletal displayed formula alkoxy molecules diols triols cyclic cycloalcohols isomers cycloalkanols molecules epoxy compounds GCE AS A2 organic ...

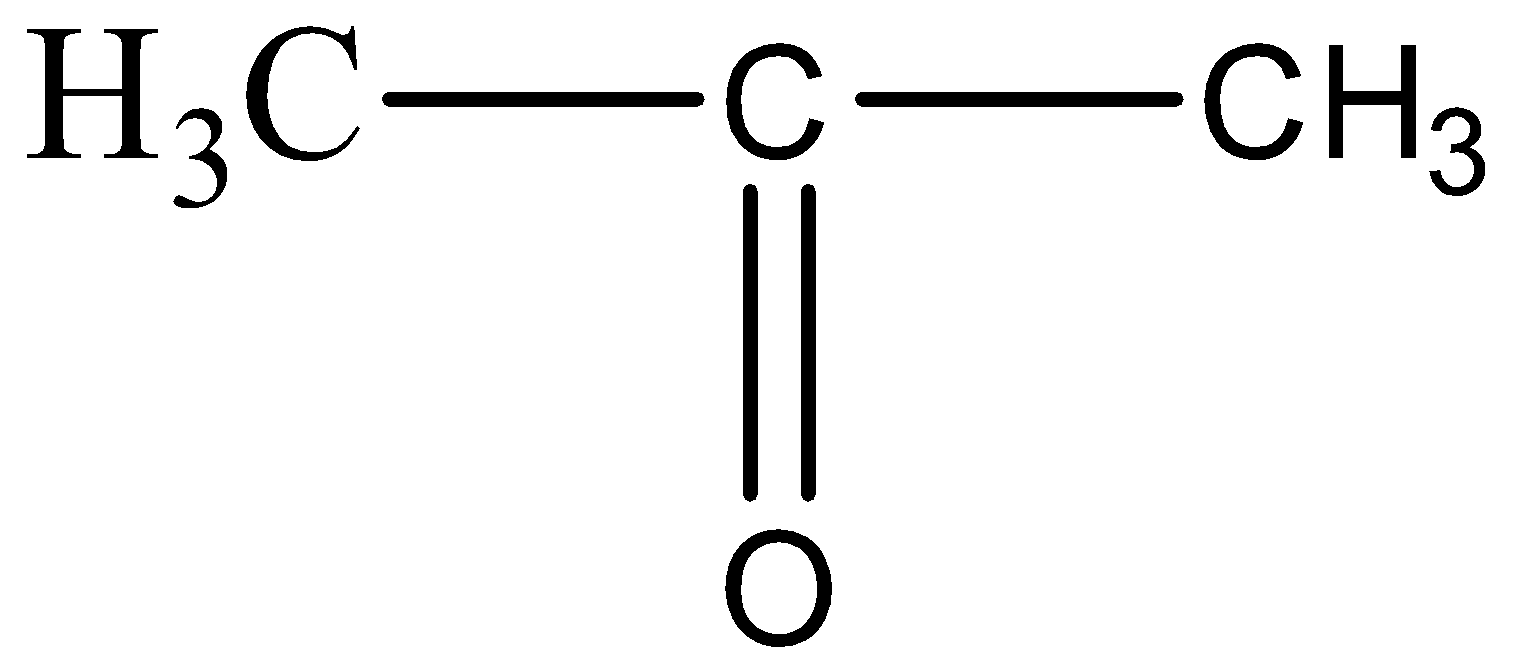
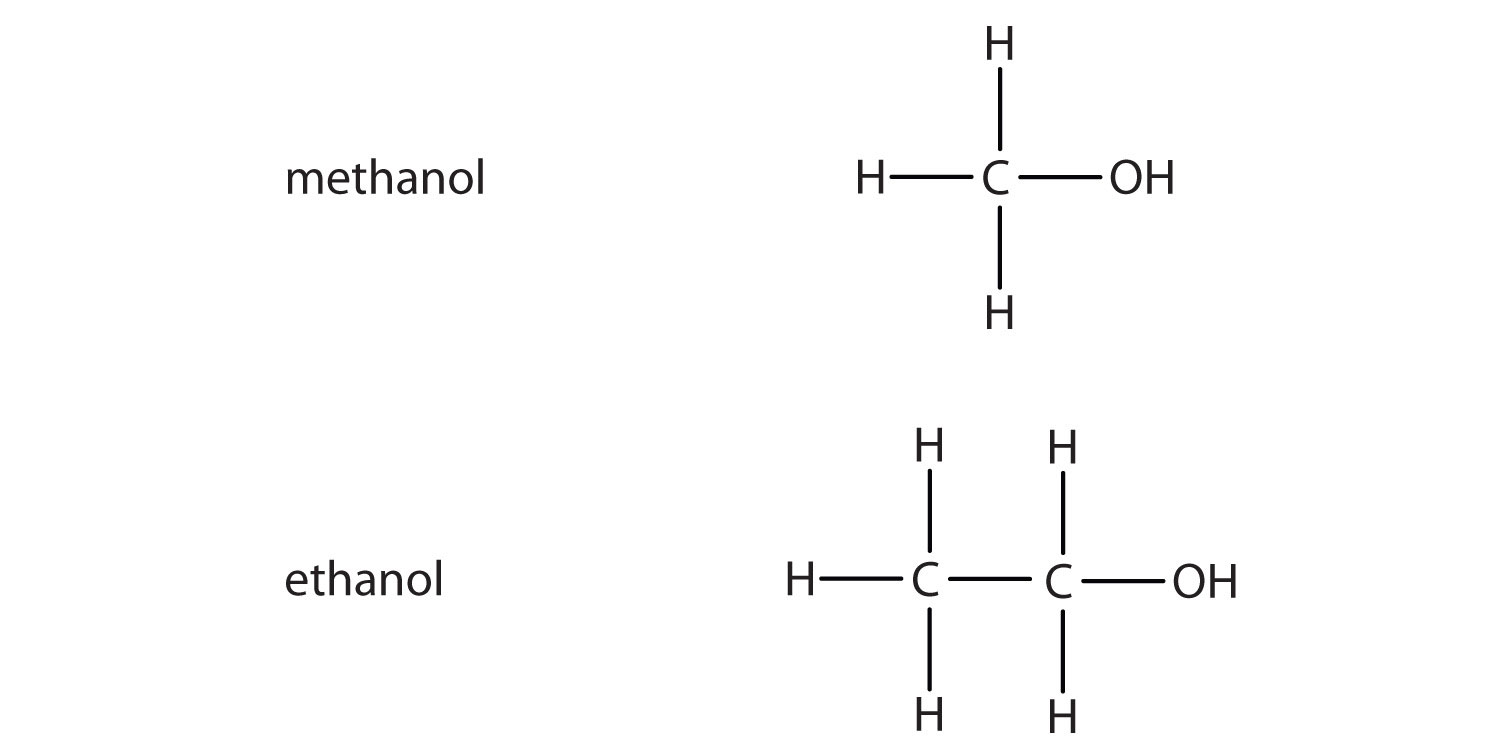
What are alcohols? An alcohol contains a hydroxyl group (—OH) attached to a carbon chain. A phenol contains a hydroxyl group (—OH) attached to a benzene. - ppt download